Ultrastructural analysis of vascular calcifications in uremia
- PMID: 20203159
- PMCID: PMC2844300
- DOI: 10.1681/ASN.2009080829
Ultrastructural analysis of vascular calcifications in uremia
Abstract
Accelerated intimal and medial calcification and sclerosis accompany the increased cardiovascular mortality of dialysis patients, but the pathomechanisms initiating microcalcifications of the media are largely unknown. In this study, we systematically investigated the ultrastructural properties of medial calcifications from patients with uremia. We collected iliac artery segments from 30 dialysis patients before kidney transplantation and studied them by radiography, microcomputed tomography, light microscopy, and transmission electron microscopy including electron energy loss spectrometry, energy dispersive spectroscopy, and electron diffraction. In addition, we performed synchrotron x-ray analyses and immunogold labeling to detect inhibitors of calcification. Von Kossa staining revealed calcification of 53% of the arteries. The diameter of these microcalcifications ranged from 20 to 500 nm, with a core-shell structure consisting of up to three layers (subshells). Many of the calcifications consisted of 2- to 10-nm nanocrystals and showed a hydroxyapatite and whitlockite crystalline structure and mineral phase. Immunogold labeling of calcification foci revealed the calcification inhibitors fetuin-A, osteopontin, and matrix gla protein. These observations suggest that uremic microcalcifications originate from nanocrystals, are chemically diverse, and intimately associate with proteinaceous inhibitors of calcification. Furthermore, considering the core-shell structure of the calcifications, apoptotic bodies or matrix vesicles may serve as a calcification nidus.
Figures





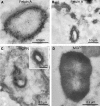
References
-
- Patient mortality and survival. United States Renal Data System. Am J Kidney Dis 32[ Suppl 1]: S69– S80, 1998 - PubMed
-
- Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM: Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension 38: 938– 942, 2001 - PubMed
-
- Schlieper G, Kruger T, Djuric Z, Damjanovic T, Markovic N, Schurgers LJ, Brandenburg VM, Westenfeld R, Dimkovic S, Ketteler M, Grootendorst DC, Dekker FW, Floege J, Dimkovic N: Vascular access calcification predicts mortality in hemodialysis patients. Kidney Int 74: 1582– 1587, 2008 - PubMed
-
- Ketteler M, Westenfeld R, Schlieper G, Brandenburg V: Pathogenesis of vascular calcification in dialysis patients. Clin Exp Nephrol 9: 265– 270, 2005 - PubMed
-
- Moe SM, Reslerova M, Ketteler M, O'Neill K, Duan D, Koczman J, Westenfeld R, Jahnen-Dechent W, Chen NX: Role of calcification inhibitors in the pathogenesis of vascular calcification in chronic kidney disease (CKD). Kidney Int 67: 2295– 2304, 2005 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

