Type 3 deiodinase, a thyroid-hormone-inactivating enzyme, controls survival and maturation of cone photoreceptors
- PMID: 20203194
- PMCID: PMC2843520
- DOI: 10.1523/JNEUROSCI.5267-09.2010
Type 3 deiodinase, a thyroid-hormone-inactivating enzyme, controls survival and maturation of cone photoreceptors
Abstract
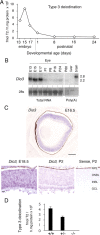
Maturation of the mammalian nervous system requires adequate provision of thyroid hormone and mechanisms that enhance tissue responses to the hormone. Here, we report that the development of cones, the photoreceptors for daylight and color vision, requires protection from thyroid hormone by type 3 deiodinase, a thyroid hormone-inactivating enzyme. Type 3 deiodinase, encoded by Dio3, is expressed in the immature mouse retina. In Dio3(-/-) mice, approximately 80% of cones are lost through neonatal cell death. Cones that express opsin photopigments for response to both short (S) and medium-long (M) wavelength light are lost. Rod photoreceptors, which mediate dim light vision, remain essentially intact. Excessive thyroid hormone in wild-type pups also eliminates cones. Cone loss is mediated by cone-specific thyroid hormone receptor beta2 (TRbeta2) as deletion of TRbeta2 rescues cones in Dio3(-/-) mice. However, rescued cones respond to short but not longer wavelength light because TRbeta2 under moderate hormonal stimulation normally induces M opsin and controls the patterning of M and S opsins over the retina. The results suggest that type 3 deiodinase limits hormonal exposure of the cone to levels that safeguard both cone survival and the patterning of opsins that is required for cone function.
Figures





References
-
- Allison WT, Dann SG, Veldhoen KM, Hawryshyn CW. Degeneration and regeneration of ultraviolet cone photoreceptors during development in rainbow trout. J Comp Neurol. 2006;499:702–715. - PubMed
-
- Applebury ML, Antoch MP, Baxter LC, Chun LL, Falk JD, Farhangfar F, Kage K, Krzystolik MG, Lyass LA, Robbins JT. The murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron. 2000;27:513–523. - PubMed
-
- Becker KB, Stephens KC, Davey JC, Schneider MJ, Galton VA. The type 2 and type 3 iodothyronine deiodinases play important roles in coordinating development in Rana catesbeiana tadpoles. Endocrinology. 1997;138:2989–2997. - PubMed
-
- Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;23:38–89. - PubMed
-
- Cao XY, Jiang XM, Dou ZH, Rakeman MA, Zhang ML, O'Donnell K, Ma T, Amette K, DeLong N, DeLong GR. Timing of vulnerability of the brain to iodine deficiency in endemic cretinism. N Engl J Med. 1994;331:1739–1744. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
