Impact of actin filament stabilization on adult hippocampal and olfactory bulb neurogenesis
- PMID: 20203201
- PMCID: PMC6634121
- DOI: 10.1523/JNEUROSCI.4231-09.2010
Impact of actin filament stabilization on adult hippocampal and olfactory bulb neurogenesis
Abstract
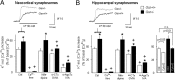
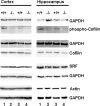
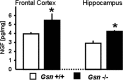
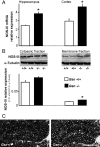
Rearrangement of the actin cytoskeleton is essential for dynamic cellular processes. Decreased actin turnover and rigidity of cytoskeletal structures have been associated with aging and cell death. Gelsolin is a Ca(2+)-activated actin-severing protein that is widely expressed throughout the adult mammalian brain. Here, we used gelsolin-deficient (Gsn(-/-)) mice as a model system for actin filament stabilization. In Gsn(-/-) mice, emigration of newly generated cells from the subventricular zone into the olfactory bulb was slowed. In vitro, gelsolin deficiency did not affect proliferation or neuronal differentiation of adult neural progenitors cells (NPCs) but resulted in retarded migration. Surprisingly, hippocampal neurogenesis was robustly induced by gelsolin deficiency. The ability of NPCs to intrinsically sense excitatory activity and thereby implement coupling between network activity and neurogenesis has recently been established. Depolarization-induced [Ca(2+)](i) increases and exocytotic neurotransmitter release were enhanced in Gsn(-/-) synaptosomes. Importantly, treatment of Gsn(-/-) synaptosomes with mycotoxin cytochalasin D, which, like gelsolin, produces actin disassembly, decreased enhanced Ca(2+) influx and subsequent exocytotic norepinephrine release to wild-type levels. Similarly, depolarization-induced glutamate release from Gsn(-/-) brain slices was increased. Furthermore, increased hippocampal neurogenesis in Gsn(-/-) mice was associated with a special microenvironment characterized by enhanced density of perfused vessels, increased regional cerebral blood flow, and increased endothelial nitric oxide synthase (NOS-III) expression in hippocampus. Together, reduced filamentous actin turnover in presynaptic terminals causes increased Ca(2+) influx and, subsequently, elevated exocytotic neurotransmitter release acting on neural progenitors. Increased neurogenesis in Gsn(-/-) hippocampus is associated with a special vascular niche for neurogenesis.
Figures



References
-
- Baldwin ML, Rostas JA, Sim AT. Two modes of exocytosis from synaptosomes are differentially regulated by protein phosphatase types 2A and 2B. J Neurochem. 2003;85:1190–1199. - PubMed
-
- Bamburg JR. Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu Rev Cell Dev Biol. 1999;15:185–230. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
