Dynamics of synaptic transmission between fast-spiking interneurons and striatal projection neurons of the direct and indirect pathways
- PMID: 20203210
- PMCID: PMC6634087
- DOI: 10.1523/JNEUROSCI.5139-09.2010
Dynamics of synaptic transmission between fast-spiking interneurons and striatal projection neurons of the direct and indirect pathways
Abstract
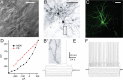
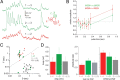
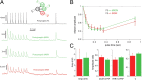
The intrastriatal microcircuit is a predominantly inhibitory GABAergic network comprised of a majority of projection neurons [medium spiny neurons (MSNs)] and a minority of interneurons. The connectivity within this microcircuit is divided into two main categories: lateral connectivity between MSNs, and inhibition mediated by interneurons, in particular fast spiking (FS) cells. To understand the operation of striatum, it is essential to have a good description of the dynamic properties of these respective pathways and how they affect different types of striatal projection neurons. We recorded from neuronal pairs, triplets, and quadruplets in slices of rat and mouse striatum and analyzed the dynamics of synaptic transmission between MSNs and FS cells. Retrograde fluorescent labeling and transgenic EGFP (enhanced green fluorescent protein) mice were used to distinguish between MSNs of the direct (striatonigral) and indirect (striatopallidal) pathways. Presynaptic neurons were stimulated with trains of action potentials, and activity-dependent depression and facilitation of synaptic efficacy was recorded from postsynaptic neurons. We found that FS cells provide a strong and homogeneously depressing inhibition of both striatonigral and striatopallidal MSN types. Moreover, individual FS cells are connected to MSNs of both types. In contrast, both MSN types receive sparse and variable, depressing and facilitating synaptic transmission from nearby MSNs. The connection probability was higher for pairs with presynaptic striatopallidal MSNs; however, the variability in synaptic dynamics did not depend on the types of interconnected MSNs. The differences between the two inhibitory pathways were clear in both species and at different developmental stages. Our findings show that the two intrastriatal inhibitory pathways have fundamentally different dynamic properties that are, however, similarly applied to both direct and indirect striatal projections.
Figures



Similar articles
-
Target selectivity of feedforward inhibition by striatal fast-spiking interneurons.J Neurosci. 2013 Jan 23;33(4):1678-83. doi: 10.1523/JNEUROSCI.3572-12.2013. J Neurosci. 2013. PMID: 23345240 Free PMC article.
-
Differential modulation of excitatory and inhibitory striatal synaptic transmission by histamine.J Neurosci. 2011 Oct 26;31(43):15340-51. doi: 10.1523/JNEUROSCI.3144-11.2011. J Neurosci. 2011. PMID: 22031880 Free PMC article.
-
Distinct roles of GABAergic interneurons in the regulation of striatal output pathways.J Neurosci. 2010 Feb 10;30(6):2223-34. doi: 10.1523/JNEUROSCI.4870-09.2010. J Neurosci. 2010. PMID: 20147549 Free PMC article.
-
Dopamine-modulated dynamic cell assemblies generated by the GABAergic striatal microcircuit.Neural Netw. 2009 Oct;22(8):1174-88. doi: 10.1016/j.neunet.2009.07.018. Epub 2009 Jul 19. Neural Netw. 2009. PMID: 19646846 Review.
-
Development of striatal fast-spiking GABAergic interneurons.Prog Brain Res. 2007;160:261-72. doi: 10.1016/S0079-6123(06)60015-0. Prog Brain Res. 2007. PMID: 17499119 Review.
Cited by
-
Striatal microcircuitry and movement disorders.Trends Neurosci. 2012 Sep;35(9):557-64. doi: 10.1016/j.tins.2012.06.008. Epub 2012 Jul 31. Trends Neurosci. 2012. PMID: 22858522 Free PMC article. Review.
-
Target selectivity of feedforward inhibition by striatal fast-spiking interneurons.J Neurosci. 2013 Jan 23;33(4):1678-83. doi: 10.1523/JNEUROSCI.3572-12.2013. J Neurosci. 2013. PMID: 23345240 Free PMC article.
-
Temporal convergence of dynamic cell assemblies in the striato-pallidal network.J Neurosci. 2012 Feb 15;32(7):2473-84. doi: 10.1523/JNEUROSCI.4830-11.2012. J Neurosci. 2012. PMID: 22396421 Free PMC article.
-
Different corticostriatal integration in spiny projection neurons from direct and indirect pathways.Front Syst Neurosci. 2010 Jun 10;4:15. doi: 10.3389/fnsys.2010.00015. eCollection 2010. Front Syst Neurosci. 2010. PMID: 20589098 Free PMC article.
-
Dynamic postnatal development of the cellular and circuit properties of striatal D1 and D2 spiny projection neurons.J Physiol. 2019 Nov;597(21):5265-5293. doi: 10.1113/JP278416. Epub 2019 Oct 10. J Physiol. 2019. PMID: 31531863 Free PMC article.
References
-
- Banitt Y, Martin KA, Segev I. Depressed responses of facilitatory synapses. J Neurophysiol. 2005;94:865–870. - PubMed
-
- Barnes TD, Kubota Y, Hu D, Jin DZ, Graybiel AM. Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories. Nature. 2005;437:1158–1161. - PubMed
-
- Bennett BD, Bolam JP. Synaptic input and output of parvalbumin-immunoreactive neurons in the neostriatum of the rat. Neuroscience. 1994;62:707–719. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
