A role for RhoB in synaptic plasticity and the regulation of neuronal morphology
- PMID: 20203211
- PMCID: PMC6634083
- DOI: 10.1523/JNEUROSCI.5386-09.2010
A role for RhoB in synaptic plasticity and the regulation of neuronal morphology
Erratum in
-
Correction: McNair et al. "A Role for RhoB in Synaptic Plasticity and the Regulation of Neuronal Morphology".J Neurosci. 2017 Sep 20;37(38):9346. doi: 10.1523/JNEUROSCI.2529-17.2017. J Neurosci. 2017. PMID: 31335926 Free PMC article.
Abstract
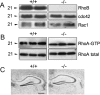
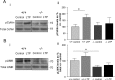
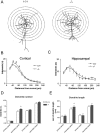
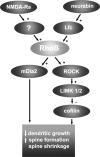
Actin-rich dendritic spines are the locus of excitatory synaptic transmission and plastic events such as long-term potentiation (LTP). Morphological plasticity of spines accompanies activity-dependent changes in synaptic strength. Several Rho GTPase family members are implicated in regulating neuronal and, in particular, spine structure via actin and the actin-binding protein cofilin. However, despite expression in hippocampus and cortex, its ability to modulate actin-regulatory proteins, and its induction during aging, RhoB has been relatively neglected. We previously demonstrated that LTP is associated with specific RhoB activation. Here, we further examined its role in synaptic function using mice with genetic deletion of the RhoB GTPase (RhoB(-/-) mice). Normal basal synaptic transmission accompanied reduced paired-pulse facilitation and post-tetanic potentiation in the hippocampus of RhoB(-/-) mice. Early phase LTP was significantly reduced in RhoB(-/-) animals, whereas the later phase was unaffected. In wild-type mice (RhoB(+/+)), Western blot analysis of potentiated hippocampus showed significant increases in phosphorylated cofilin relative to nonpotentiated slices, which were dramatically impaired in RhoB(-/-) slices. There was also a deficit in phosphorylated Lim kinase levels in the hippocampus from RhoB(-/-) mice. Morphological analysis suggested that lack of RhoB resulted in increased dendritic branching and decreased spine number. Furthermore, an increase in the proportion of stubby relative to thin spines was observed. Moreover, spines demonstrated increased length along with increased head and neck widths. These data implicate RhoB in cofilin regulation and dendritic and spine morphology, highlighting its importance in synaptic plasticity at a structural and functional level.
Figures




Similar articles
-
PI3K couples long-term synaptic potentiation with cofilin recruitment and actin polymerization in dendritic spines via its regulatory subunit p85α.Cell Mol Life Sci. 2024 Aug 19;81(1):358. doi: 10.1007/s00018-024-05394-x. Cell Mol Life Sci. 2024. PMID: 39158722 Free PMC article.
-
Selective regulation of GluA subunit synthesis and AMPA receptor-mediated synaptic function and plasticity by the translation repressor 4E-BP2 in hippocampal pyramidal cells.J Neurosci. 2013 Jan 30;33(5):1872-86. doi: 10.1523/JNEUROSCI.3264-12.2013. J Neurosci. 2013. PMID: 23365227 Free PMC article.
-
Chemically induced long-term potentiation increases the number of perforated and complex postsynaptic densities but does not alter dendritic spine volume in CA1 of adult mouse hippocampal slices.Eur J Neurosci. 2005 Jun;21(12):3368-78. doi: 10.1111/j.1460-9568.2005.04174.x. Eur J Neurosci. 2005. PMID: 16026474
-
Role of Drebrin in Synaptic Plasticity.Adv Exp Med Biol. 2017;1006:183-201. doi: 10.1007/978-4-431-56550-5_11. Adv Exp Med Biol. 2017. PMID: 28865021 Review.
-
Regulation of spine morphology and synaptic function by LIMK and the actin cytoskeleton.Rev Neurosci. 2003;14(3):233-40. doi: 10.1515/revneuro.2003.14.3.233. Rev Neurosci. 2003. PMID: 14513866 Review.
Cited by
-
Determination of dendritic spine morphology by the striatin scaffold protein STRN4 through interaction with the phosphatase PP2A.J Biol Chem. 2017 Jun 9;292(23):9451-9464. doi: 10.1074/jbc.M116.772442. Epub 2017 Apr 25. J Biol Chem. 2017. PMID: 28442576 Free PMC article.
-
Rho family GTPases: key players in neuronal development, neuronal survival, and neurodegeneration.Front Cell Neurosci. 2014 Oct 7;8:314. doi: 10.3389/fncel.2014.00314. eCollection 2014. Front Cell Neurosci. 2014. PMID: 25339865 Free PMC article. Review.
-
Deletion of JMJD2B in neurons leads to defective spine maturation, hyperactive behavior and memory deficits in mouse.Transl Psychiatry. 2016 Mar 29;6(3):e766. doi: 10.1038/tp.2016.31. Transl Psychiatry. 2016. PMID: 27023172 Free PMC article.
-
KCTD10 p.C124W variant contributes to schizophrenia by attenuating LLPS-mediated synapse formation.Proc Natl Acad Sci U S A. 2024 Nov 26;121(48):e2400464121. doi: 10.1073/pnas.2400464121. Epub 2024 Nov 20. Proc Natl Acad Sci U S A. 2024. PMID: 39565307 Free PMC article.
-
ADF/cofilin: a crucial regulator of synapse physiology and behavior.Cell Mol Life Sci. 2015 Sep;72(18):3521-9. doi: 10.1007/s00018-015-1941-z. Epub 2015 Jun 3. Cell Mol Life Sci. 2015. PMID: 26037722 Free PMC article. Review.
References
-
- Bailey CH, Kandel ER. Structural changes accompanying memory storage. Annu Rev Physiol. 1993;55:397–426. - PubMed
-
- Bourne J, Harris KM. Do thin spines learn to be mushroom spines that remember? Curr Opin Neurobiol. 2007;17:381–386. - PubMed
-
- Brabeck C, Mittelbronn M, Bekure K, Meyermann R, Schluesener HJ, Schwab JM. Effect of focal cerebral infarctions on lesional RhoA and RhoB expression. Arch Neurol. 2003;60:1245–1249. - PubMed
-
- Brabeck C, Beschorner R, Conrad S, Mittelbronn M, Bekure K, Meyermann R, Schluesener HJ, Schwab JM. Lesional expression of RhoA and RhoB following traumatic brain injury in humans. J Neurotrauma. 2004;21:697–706. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
