Out of the darkness and into the light: bright field in situ hybridisation for delineation of ERBB2 (HER2) status in breast carcinoma
- PMID: 20203220
- PMCID: PMC2921277
- DOI: 10.1136/jcp.2009.062760
Out of the darkness and into the light: bright field in situ hybridisation for delineation of ERBB2 (HER2) status in breast carcinoma
Abstract
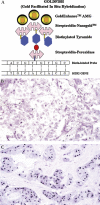
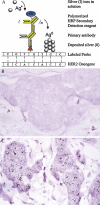
Assessment of ERBB2 (HER2) status in breast carcinomas has become critical in determining response to the humanised monoclonal antibody trastuzumab. The current joint College of American Pathologists and the American Society of Clinical Oncology guidelines for the evaluation of HER2 status in breast carcinoma involve testing by immunohistochemistry and fluorescence in situ hybridisation (FISH). However, neither of these modalities is without limitations. Novel bright field in situ hybridisation techniques continue to provide viable alternatives to FISH testing. While these techniques are not limited to evaluation of the HER2 gene, the extensive number of studies comparing bright field in situ techniques with other methods of assessing HER2 status allow a robust evaluation of this approach. Analysis of the literature demonstrates that, when used to assess HER2 gene status, bright field in situ hybridisation demonstrates excellent concordance with FISH results. The average percentage agreement in an informal analysis of studies comparing HER2 amplification by chromogenic in situ hybridisation with FISH was 96% (SD 4%); kappa coefficients ranged from 0.76 to 1.0. Although a much smaller number of studies are available for review, similar levels of concordance have been reported in studies comparing HER2 amplification by methods employing metallography (silver in situ hybridisation) with FISH. A summary of the advancements in bright field in situ hybridisation, with focus on those techniques with clinical applications of interest to the practicing pathologist, is presented.
Conflict of interest statement
Figures


Similar articles
-
Comparison of automated silver enhanced in situ hybridisation (SISH) and fluorescence ISH (FISH) for the validation of HER2 gene status in breast carcinoma according to the guidelines of the American Society of Clinical Oncology and the College of American Pathologists.Virchows Arch. 2007 Jul;451(1):19-25. doi: 10.1007/s00428-007-0424-5. Epub 2007 Jun 12. Virchows Arch. 2007. PMID: 17562074
-
Bright-field in situ hybridization for HER2 gene amplification in breast cancer using tissue microarrays: correlation between chromogenic (CISH) and automated silver-enhanced (SISH) methods with patient outcome.Diagn Mol Pathol. 2009 Jun;18(2):88-95. doi: 10.1097/PDM.0b013e31816f6374. Diagn Mol Pathol. 2009. PMID: 19430296
-
Silver in situ hybridization (SISH) for determination of HER2 gene status in breast carcinoma: comparison with FISH and assessment of interobserver reproducibility.Am J Surg Pathol. 2010 Jun;34(6):767-76. doi: 10.1097/PAS.0b013e3181d96231. Am J Surg Pathol. 2010. PMID: 20421783
-
Emerging technologies for assessing HER2 amplification.Am J Clin Pathol. 2009 Oct;132(4):539-48. doi: 10.1309/AJCPV2I0HGPMGBSQ. Am J Clin Pathol. 2009. PMID: 19762531 Review.
-
HER2 assessment by silver in situ hybridization: where are we now?Expert Rev Mol Diagn. 2015 Mar;15(3):385-98. doi: 10.1586/14737159.2015.992416. Epub 2015 Jan 12. Expert Rev Mol Diagn. 2015. PMID: 25578771 Review.
Cited by
-
Optimizing Ventana chromogenic dual in-situ hybridization for mucinous epithelial ovarian cancer.BMC Res Notes. 2013 Dec 28;6:562. doi: 10.1186/1756-0500-6-562. BMC Res Notes. 2013. PMID: 24373486 Free PMC article.
-
Comparison of automated and manual FISH for evaluation of HER2 gene status on breast carcinoma core biopsies.BMC Clin Pathol. 2013 Apr 20;13:13. doi: 10.1186/1472-6890-13-13. BMC Clin Pathol. 2013. PMID: 23601823 Free PMC article.
-
The clinical and biological significance of HER2 over-expression in breast ductal carcinoma in situ: a large study from a single institution.Br J Cancer. 2019 May;120(11):1075-1082. doi: 10.1038/s41416-019-0436-3. Epub 2019 May 8. Br J Cancer. 2019. PMID: 31065110 Free PMC article.
-
Copy number gains of FGFR1 and 3q chromosome in squamous cell carcinoma of the lung.Transl Lung Cancer Res. 2013 Apr;2(2):101-11. doi: 10.3978/j.issn.2218-6751.2013.03.05. Transl Lung Cancer Res. 2013. PMID: 25806221 Free PMC article. Review.
-
Not All Next Generation Sequencing Diagnostics are Created Equal: Understanding the Nuances of Solid Tumor Assay Design for Somatic Mutation Detection.Cancers (Basel). 2015 Jul 17;7(3):1313-32. doi: 10.3390/cancers7030837. Cancers (Basel). 2015. PMID: 26193321 Free PMC article. Review.
References
-
- Watson JD, Crick FH. Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature 1953;171:737–8 - PubMed
-
- Tjio JH, Levan A. The chromosome number of man. Hereditas 1956;42:1–6
-
- de Jong H. Visualizing DNA domains and sequences by microscopy: a fifty-year history of molecular cytogenetics. Genome 2003;46:943–6 - PubMed
-
- Schildkraut CL, Marmur J, Doty P. The formation of hybrid DNA molecules and their use in studies of DNA homologies. J Mol Biol 1961;3:595–617 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
