S-nitrosylation in cardiovascular signaling
- PMID: 20203313
- PMCID: PMC2891248
- DOI: 10.1161/CIRCRESAHA.109.207381
S-nitrosylation in cardiovascular signaling
Abstract
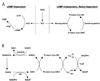
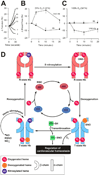
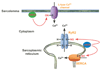
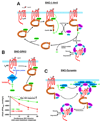
Well over 2 decades have passed since the endothelium-derived relaxation factor was reported to be the gaseous molecule nitric oxide (NO). Although soluble guanylyl cyclase (which generates cyclic guanosine monophosphate, cGMP) was the first identified receptor for NO, it has become increasingly clear that NO exerts a ubiquitous influence in a cGMP-independent manner. In particular, many, if not most, effects of NO are mediated by S-nitrosylation, the covalent modification of a protein cysteine thiol by an NO group to generate an S-nitrosothiol (SNO). Moreover, within the current framework of NO biology, endothelium-derived relaxation factor activity (ie, G protein-coupled receptor-mediated, or shear-induced endothelium-derived NO bioactivity) is understood to involve a central role for SNOs, acting both as second messengers and signal effectors. Furthermore, essential roles for S-nitrosylation have been implicated in virtually all major functions of NO in the cardiovascular system. Here, we review the basic biochemistry of S-nitrosylation (and denitrosylation), discuss the role of S-nitrosylation in the vascular and cardiac functions of NO, and identify current and potential clinical applications.
Figures

Comment in
-
Nitric oxide and the ryanodine receptor Ca-release channel.Circ Res. 2010 Jul 9;107(1):e1; author reply e2-3. doi: 10.1161/CIRCRESAHA.110.221887. Circ Res. 2010. PMID: 20616330 No abstract available.
Similar articles
-
Protein S-Nitrosylation: Determinants of Specificity and Enzymatic Regulation of S-Nitrosothiol-Based Signaling.Antioxid Redox Signal. 2019 Apr 1;30(10):1331-1351. doi: 10.1089/ars.2017.7403. Epub 2018 Jan 10. Antioxid Redox Signal. 2019. PMID: 29130312 Free PMC article. Review.
-
Signaling by S-nitrosylation in the heart.J Mol Cell Cardiol. 2014 Aug;73:18-25. doi: 10.1016/j.yjmcc.2014.01.003. Epub 2014 Jan 16. J Mol Cell Cardiol. 2014. PMID: 24440455 Free PMC article. Review.
-
[Regulation of vascular function by nitric oxide-related S-nitrosylation].Sheng Li Xue Bao. 2017 Oct 25;69(5):557-570. Sheng Li Xue Bao. 2017. PMID: 29063104 Review. Chinese.
-
S-nitrosothiols and the S-nitrosoproteome of the cardiovascular system.Antioxid Redox Signal. 2013 Jan 20;18(3):270-87. doi: 10.1089/ars.2012.4744. Epub 2012 Sep 5. Antioxid Redox Signal. 2013. PMID: 22770551 Free PMC article. Review.
-
Vascular nitric oxide: Beyond eNOS.J Pharmacol Sci. 2015 Oct;129(2):83-94. doi: 10.1016/j.jphs.2015.09.002. Epub 2015 Sep 28. J Pharmacol Sci. 2015. PMID: 26499181 Review.
Cited by
-
Quantitative proteomics reveals differential regulation of protein expression in recipient myocardium after trilineage cardiovascular cell transplantation.Proteomics. 2015 Aug;15(15):2560-7. doi: 10.1002/pmic.201500131. Proteomics. 2015. PMID: 26033914 Free PMC article.
-
S-Nitroso-L-Cysteine Stereoselectively Blunts the Deleterious Effects of Fentanyl on Breathing While Augmenting Antinociception in Freely-Moving Rats.Front Pharmacol. 2022 May 26;13:892307. doi: 10.3389/fphar.2022.892307. eCollection 2022. Front Pharmacol. 2022. PMID: 35721204 Free PMC article.
-
Nitric oxide signalling in cardiovascular health and disease.Nat Rev Cardiol. 2018 May;15(5):292-316. doi: 10.1038/nrcardio.2017.224. Epub 2018 Feb 1. Nat Rev Cardiol. 2018. PMID: 29388567 Review.
-
ALDH2 activator inhibits increased myocardial infarction injury by nitroglycerin tolerance.Sci Transl Med. 2011 Nov 2;3(107):107ra111. doi: 10.1126/scitranslmed.3002067. Sci Transl Med. 2011. PMID: 22049071 Free PMC article.
-
Heterologous down-regulation of angiotensin type 1 receptors by purinergic P2Y2 receptor stimulation through S-nitrosylation of NF-kappaB.Proc Natl Acad Sci U S A. 2011 Apr 19;108(16):6662-7. doi: 10.1073/pnas.1017640108. Epub 2011 Apr 4. Proc Natl Acad Sci U S A. 2011. PMID: 21464294 Free PMC article.
References
-
- Guyton AC, Coleman TG, Granger HJ. Circulation: overall regulation. Annu Rev Physiol. 1972;34:13–46. - PubMed
-
- McMahon TJ, Moon RE, Luschinger BP, Carraway MS, Stone AE, Stolp BW, Gow AJ, Pawloski JR, Watke P, Singel DJ, Piantadosi CA, Stamler JS. Nitric oxide in the human respiratory cycle. Nat Med. 2002;8:711–717. - PubMed
-
- Dzik S. Nitric oxide: nature's third respiratory gas. Transfusion. 2002;42:1532–1533. - PubMed
-
- Bredt DS, Snyder SH. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem. 1994;63:175–195. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

