A coupled SYSTEM of intracellular Ca2+ clocks and surface membrane voltage clocks controls the timekeeping mechanism of the heart's pacemaker
- PMID: 20203315
- PMCID: PMC2837285
- DOI: 10.1161/CIRCRESAHA.109.206078
A coupled SYSTEM of intracellular Ca2+ clocks and surface membrane voltage clocks controls the timekeeping mechanism of the heart's pacemaker
Abstract
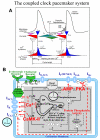
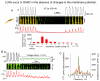
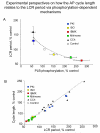
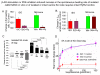
Ion channels on the surface membrane of sinoatrial nodal pacemaker cells (SANCs) are the proximal cause of an action potential. Each individual channel type has been thoroughly characterized under voltage clamp, and the ensemble of the ion channel currents reconstructed in silico generates rhythmic action potentials. Thus, this ensemble can be envisioned as a surface "membrane clock" (M clock). Localized subsarcolemmal Ca(2+) releases are generated by the sarcoplasmic reticulum via ryanodine receptors during late diastolic depolarization and are referred to as an intracellular "Ca(2+) clock," because their spontaneous occurrence is periodic during voltage clamp or in detergent-permeabilized SANCs, and in silico as well. In spontaneously firing SANCs, the M and Ca(2+) clocks do not operate in isolation but work together via numerous interactions modulated by membrane voltage, subsarcolemmal Ca(2+), and protein kinase A and CaMKII-dependent protein phosphorylation. Through these interactions, the 2 subsystem clocks become mutually entrained to form a robust, stable, coupled-clock system that drives normal cardiac pacemaker cell automaticity. G protein-coupled receptors signaling creates pacemaker flexibility, ie, effects changes in the rhythmic action potential firing rate, by impacting on these very same factors that regulate robust basal coupled-clock system function. This review examines evidence that forms the basis of this coupled-clock system concept in cardiac SANCs.
Figures




References
-
- Irisawa H, Brown HF, Giles W. Cardiac pacemaking in the sinoatrial node. Physiol Rev. 1993;73:197–227. - PubMed
-
- Baruscotti M, Barbuti A, Bucchi A. The cardiac pacemaker current. J Mol Cell Cardiol. 2009 (in print) - PubMed
-
- Mangoni ME, Nargeot J. Genesis and regulation of the heart automaticity. Physiol Rev. 2008;88:919–982. - PubMed
-
- Wilders R. Computer modelling of the sinoatrial node. Med Biol Eng Comput. 2007;45:189–207. - PubMed
-
- Rubenstein DS, Lipsius SL. Mechanisms of automaticity in subsidiary pacemakers from cat right atrium. Circ Res. 1989;64:648–657. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

