SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation
- PMID: 20203611
- PMCID: PMC2841477
- DOI: 10.1038/nature08778
SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation
Abstract
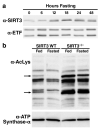
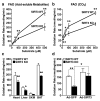
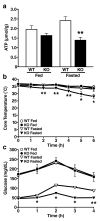
Sirtuins are NAD(+)-dependent protein deacetylases. They mediate adaptive responses to a variety of stresses, including calorie restriction and metabolic stress. Sirtuin 3 (SIRT3) is localized in the mitochondrial matrix, where it regulates the acetylation levels of metabolic enzymes, including acetyl coenzyme A synthetase 2 (refs 1, 2). Mice lacking both Sirt3 alleles appear phenotypically normal under basal conditions, but show marked hyperacetylation of several mitochondrial proteins. Here we report that SIRT3 expression is upregulated during fasting in liver and brown adipose tissues. During fasting, livers from mice lacking SIRT3 had higher levels of fatty-acid oxidation intermediate products and triglycerides, associated with decreased levels of fatty-acid oxidation, compared to livers from wild-type mice. Mass spectrometry of mitochondrial proteins shows that long-chain acyl coenzyme A dehydrogenase (LCAD) is hyperacetylated at lysine 42 in the absence of SIRT3. LCAD is deacetylated in wild-type mice under fasted conditions and by SIRT3 in vitro and in vivo; and hyperacetylation of LCAD reduces its enzymatic activity. Mice lacking SIRT3 exhibit hallmarks of fatty-acid oxidation disorders during fasting, including reduced ATP levels and intolerance to cold exposure. These findings identify acetylation as a novel regulatory mechanism for mitochondrial fatty-acid oxidation and demonstrate that SIRT3 modulates mitochondrial intermediary metabolism and fatty-acid use during fasting.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

