Water-filtered infrared-A (wIRA) in acute and chronic wounds
- PMID: 20204090
- PMCID: PMC2831245
- DOI: 10.3205/dgkh000137
Water-filtered infrared-A (wIRA) in acute and chronic wounds
Abstract
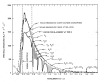
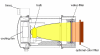
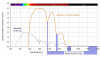
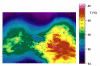
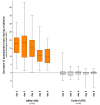
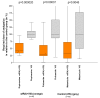
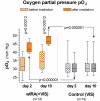
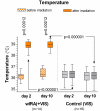
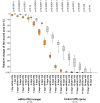
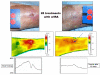
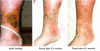
Water-filtered infrared-A (wIRA), as a special form of heat radiation with a high tissue penetration and a low thermal load to the skin surface, can improve the healing of acute and chronic wounds both by thermal and thermic as well as by non-thermal and non-thermic effects. wIRA increases tissue temperature (+2.7 degrees C at a tissue depth of 2 cm), tissue oxygen partial pressure (+32% at a tissue depth of 2 cm) and tissue perfusion. These three factors are decisive for a sufficient supply of tissue with energy and oxygen and consequently also for wound healing and infection defense. wIRA can considerably alleviate pain (without any exception during 230 irradiations) with substantially less need for analgesics (52-69% less in the groups with wIRA compared to the control groups). It also diminishes exudation and inflammation and can show positive immunomodulatory effects. The overall evaluation of the effect of irradiation as well as the wound healing and the cosmetic result (assessed on visual analogue scales) were markedly better in the group with wIRA compared to the control group. wIRA can advance wound healing (median reduction of wound size of 90% in severely burned children already after 9 days in the group with wIRA compared to 13 days in the control group; on average 18 versus 42 days until complete wound closure in chronic venous stasis ulcers) or improve an impaired wound healing (reaching wound closure and normalization of the thermographic image in otherwise recalcitrant chronic venous stasis ulcers) both in acute and in chronic wounds including infected wounds. After major abdominal surgery there was a trend in favor of the wIRA group to a lower rate of total wound infections (7% versus 15%) including late infections following discharge from hospital (0% versus 8%) and a trend towards a shorter postoperative hospital stay (9 versus 11 days). Even the normal wound healing process can be improved. The mentioned effects have been proven in six prospective studies, with most of the effects having an evidence level of Ia/Ib.wIRA represents a valuable therapy option and can generally be recommended for use in the treatment of acute as well as of chronic wounds.
Wassergefiltertes Infrarot A (wIRA) als spezielle Form der Wärmestrahlung mit hohem Eindringvermögen in das Gewebe bei geringer thermischer Oberflächenbelastung kann die Heilung akuter und chronischer Wunden sowohl über thermische und temperaturabhängige als auch über nicht-thermische und temperaturunabhängige Effekte verbessern. wIRA steigert Temperatur (+2,7°C in 2 cm Gewebetiefe) und Sauerstoffpartialdruck im Gewebe (+32% in 2 cm Gewebetiefe) sowie die Gewebedurchblutung. Diese drei Faktoren sind entscheidend für eine ausreichende Versorgung des Gewebes mit Energie und Sauerstoff und deshalb auch für Wundheilung und Infektionsabwehr.
wIRA vermag Schmerzen deutlich zu mindern (ausnahmslos bei 230 Bestrahlungen) mit bemerkenswert niedrigerem Analgetikabedarf (52–69% niedriger in den Gruppen mit wIRA verglichen mit den Kontrollgruppen) und eine erhöhte Wundsekretion und Entzündung herabzusetzen sowie positive immunmodulierende Effekte zu zeigen. Die Gesamtbeurteilung des Effekts der Bestrahlung wie auch die Wundheilung und das kosmetische Ergebnis (erhoben mittels visueller Analogskalen) waren in der Gruppe mit wIRA wesentlich besser verglichen mit der Kontrollgruppe. wIRA kann sowohl bei akuten als auch bei chronischen Wunden einschließlich infizierter Wunden die Wundheilung beschleunigen (Abnahme der Wundfläche im Median um 90% bei schwerbrandverletzten Kindern bereits nach 9 Tagen in der Gruppe mit wIRA verglichen mit 13 Tagen in der Kontrollgruppe; im Durchschnitt 18 versus 42 Tage bis zum kompletten Wundschluss bei chronischen venösen Unterschenkelulzera) oder bei stagnierender Wundheilung verbessern (mit Erreichen eines kompletten Wundschlusses und Normalisierung des thermographischen Bildes bei zuvor therapierefraktären chronischen venösen Unterschenkelulzera). Nach großen abdominalen Operationen zeigte sich ein Trend zugunsten der wIRA-Gruppe hin zu einer niedrigeren Rate von Wundinfektionen insgesamt (7% versus 15%) einschließlich später Infektionen nach der Entlassung aus dem Krankenhaus (0% versus 8%) und ein Trend hin zu einem kürzeren postoperativen Krankenhausaufenthalt (9 versus 11 Tage).
Selbst der normale Wundheilungsprozess kann verbessert werden.
Die erwähnten Effekte wurden in 6 prospektiven Studien belegt, die meisten mit einem Evidenzgrad von Ia/Ib.
wIRA stellt eine wertvolle Therapieoption dar und kann generell für die Therapie von akuten und chronischen Wunden empfohlen werden.
Keywords: absent expenditure of material; acute wounds; chronic venous stasis ulcers of the lower legs; chronic wounds; contact-free method; energy supply; immunomodulatory effects; infection defense; inflammation; infrared thermography; infrared-A radiation; oxygen supply; problem wounds; prospective, randomized, controlled, double-blind studies; quality of life; reduction of pain; thermal and non-thermal effects; thermic and non-thermic effects; thermographic image analysis; tissue blood flow; tissue oxygen partial pressure; tissue temperature; visual analogue scales (VAS); water-filtered infrared-A (wIRA); wound exudation; wound healing; wound infections.
Figures



References
-
- Hoffmann G. Principles and working mechanisms of water-filtered infrared-A (wIRA) in relation to wound healing [review] GMS Krankenhaushyg Interdiszip. 2007;2(2):Doc54. Available from: http://www.egms.de/en/journals/dgkh/2007-2/dgkh000087.shtml. - PMC - PubMed
-
- Hartel M, Illing P, Mercer JB, Lademann J, Daeschlein G, Hoffmann G. Therapy of acute wounds with water-filtered infrared-A (wIRA) [review] GMS Krankenhaushyg Interdiszip. 2007;2(2):Doc53. Available from: http://www.egms.de/en/journals/dgkh/2007-2/dgkh000086.shtml. - PMC - PubMed
-
- von Felbert V, Schumann H, Mercer JB, Strasser W, Daeschlein G, Hoffmann G. Therapy of chronic wounds with water-filtered infrared-A (wIRA) [review] [review]. GMS Krankenhaushyg Interdiszip. 2007;2(2):Doc52. (Ger). Available from: http://www.egms.de/en/journals/dgkh/2008-2/dgkh000085.shtml. - PMC - PubMed
-
- Hoffmann G. Wassergefiltertes Infrarot A (wIRA) zur Verbesserung der Wundheilung [Übersichtsarbeit] [Water-filtered infrared A (wIRA) for the improvement of wound healing [review]]. GMS Krankenhaushyg Interdiszip. 2006;1(1):Doc20. (Ger). Available from: http://www.egms.de/en/journals/dgkh/2006-1/dgkh000020.shtml.
-
- Hoffmann G. Wassergefiltertes Infrarot A (wIRA) zur Verbesserung der Wundheilung bei akuten und chronischen Wunden. [Water-filtered Infrared-A (wIRA) for the improvement of wound healing of acute and chronic wounds]. Wundmanagement. 2008;2:72–80. (Ger). Available from: http://publikationen.ub.uni-frankfurt.de/volltexte/2008/5429/
LinkOut - more resources
Full Text Sources

