Quality management for the processing of medical devices
- PMID: 20204094
- PMCID: PMC2831254
Quality management for the processing of medical devices
Abstract
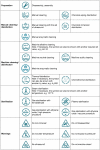
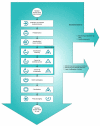
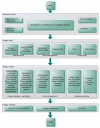
Rules on the reprocessing of medical devices were put into place in Germany in 2001. The present article explains the background situation and the provisions that are currently in force.The implementation of these statutory requirements is described using the example of the quality management system of Germany's market leader, Vanguard AG. This quality management system was successfully certified pursuant to DIN EN ISO 13485:2003 for the scope "reprocessing of medical devices", including class "critical C", in accordance with the recommendation of the Commission for Hospital Hygiene and the Prevention of Infection at the Robert-Koch-Institute (RKI) and the German Federal Institute for Drugs and Medical Devices (BfArM) on the "Hygiene requirements for reprocessing of medical devices".
In Deutschland wurde die Aufbereitung von Medizinprodukten im Jahr 2001 geregelt. In der vorliegenden Arbeit werden die Hintergründe und die bestehenden Regularien erläutert.
Am Beispiel des Qualitätsmanagementsystems der Vanguard AG als Marktführer in Deutschland wird die Umsetzung der gesetzlichen Anforderungen beschrieben. Im Ergebnis konnte das Qualitätsmanagementsystem gemäß DIN EN ISO 13485 für den Geltungsbereich der Aufbereitung von Medizinprodukten, einschließlich der Einstufung „kritisch C“, entsprechend der Empfehlung der Kommission für Krankenhaushygiene und Infektionsprävention beim Robert-Koch-Institut (RKI) und des Bundesinstitutes für Arzneimittel und Medizinprodukte (BfArM) zu den „Anforderungen an die Hygiene bei der Aufbereitung von Medizinprodukten“ zertifiziert werden.
Keywords: certification; processing of medical devices; processing procedure; processing requirements; quality management; risk management.
Figures





LinkOut - more resources
Full Text Sources
Research Materials
