Tubulin chaperone E binds microtubules and proteasomes and protects against misfolded protein stress
- PMID: 20204449
- PMCID: PMC11115895
- DOI: 10.1007/s00018-010-0308-8
Tubulin chaperone E binds microtubules and proteasomes and protects against misfolded protein stress
Abstract
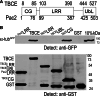
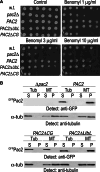
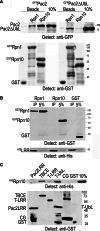
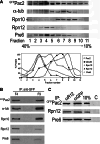
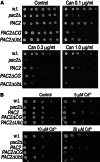
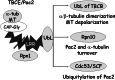
Mutation of tubulin chaperone E (TBCE) underlies hypoparathyroidism, retardation, and dysmorphism (HRD) syndrome with defective microtubule (MT) cytoskeleton. TBCE/yeast Pac2 comprises CAP-Gly, LRR (leucine-rich region), and UbL (ubiquitin-like) domains. TBCE folds alpha-tubulin and promotes alpha/beta dimerization. We show that Pac2 functions in MT dynamics: the CAP-Gly domain binds alpha-tubulin and MTs, and functions in suppression of benomyl sensitivity of pac2Delta mutants. Pac2 binds proteasomes: the LRR binds Rpn1, and the UbL binds Rpn10; the latter interaction mediates Pac2 turnover. The UbL also binds the Skp1-Cdc53-F-box (SCF) ubiquitin ligase complex; these competing interactions for the UbL may impact on MT dynamics. pac2Delta mutants are sensitive to misfolded protein stress. This is suppressed by ectopic PAC2 with both the CAP-Gly and UbL domains being essential. We propose a novel role for Pac2 in the misfolded protein stress response based on its ability to interact with both the MT cytoskeleton and the proteasomes.
Figures



Similar articles
-
Cryptic out-of-frame translational initiation of TBCE rescues tubulin formation in compound heterozygous HRD.Proc Natl Acad Sci U S A. 2006 Sep 5;103(36):13491-6. doi: 10.1073/pnas.0602798103. Epub 2006 Aug 28. Proc Natl Acad Sci U S A. 2006. PMID: 16938882 Free PMC article.
-
The structure of the complex between α-tubulin, TBCE and TBCB reveals a tubulin dimer dissociation mechanism.J Cell Sci. 2015 May 1;128(9):1824-34. doi: 10.1242/jcs.167387. Epub 2015 Apr 23. J Cell Sci. 2015. PMID: 25908846
-
A case of severe TBCE-negative hypoparathyroidism-retardation-dysmorphism syndrome: Case report and literature review.Am J Med Genet A. 2018 Aug;176(8):1768-1772. doi: 10.1002/ajmg.a.38851. Epub 2018 Jul 28. Am J Med Genet A. 2018. PMID: 30055029
-
Capturing protein tails by CAP-Gly domains.Trends Biochem Sci. 2008 Nov;33(11):535-45. doi: 10.1016/j.tibs.2008.08.006. Epub 2008 Oct 4. Trends Biochem Sci. 2008. PMID: 18835717 Review.
-
Parathyroid development and the role of tubulin chaperone E.Horm Res. 2007;67(1):12-21. doi: 10.1159/000095944. Epub 2006 Sep 27. Horm Res. 2007. PMID: 17008776 Review.
Cited by
-
Tubulin Post-Translational Modifications: The Elusive Roles of Acetylation.Biology (Basel). 2023 Apr 6;12(4):561. doi: 10.3390/biology12040561. Biology (Basel). 2023. PMID: 37106761 Free PMC article. Review.
-
The role of 26S proteasome-dependent proteolysis in the formation and restructuring of microtubule networks.Plant Signal Behav. 2012 Oct 1;7(10):1289-95. doi: 10.4161/psb.21543. Epub 2012 Aug 20. Plant Signal Behav. 2012. PMID: 22902696 Free PMC article. Review.
-
TBCE Mutations Cause Early-Onset Progressive Encephalopathy with Distal Spinal Muscular Atrophy.Am J Hum Genet. 2016 Oct 6;99(4):974-983. doi: 10.1016/j.ajhg.2016.08.006. Epub 2016 Sep 22. Am J Hum Genet. 2016. PMID: 27666369 Free PMC article.
-
The architecture of Trypanosoma brucei tubulin-binding cofactor B and implications for function.FEBS J. 2013 Jul;280(14):3270-80. doi: 10.1111/febs.12308. Epub 2013 May 24. FEBS J. 2013. PMID: 23627368 Free PMC article.
-
Faithful chaperones.Cell Mol Life Sci. 2011 Oct;68(20):3307-22. doi: 10.1007/s00018-011-0740-4. Epub 2011 Jun 8. Cell Mol Life Sci. 2011. PMID: 21655914 Free PMC article. Review.
References
-
- Parvari R, Hershkovitz E, Grossman N, Gorodischer R, Loeys B, Zecic A, Mortier G, Gregory S, Sharony R, Kambouris M, Sakati N, Meyer BF, Al Aqeel AI, Al Humaidan AK, Al Zanhrani F, Al Swaid A, Al Othman J, Diaz GA, Weiner R, Khan KT, Gordon R, Gelb BD. Mutation of TBCE causes hypoparathyroidism-retardation-dysmorphism and autosomal recessive Kenny-Caffey syndrome. Nat Genet. 2002;32:448–452. doi: 10.1038/ng1012. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

