Biogenesis of bacterial inner-membrane proteins
- PMID: 20204450
- PMCID: PMC11115511
- DOI: 10.1007/s00018-010-0303-0
Biogenesis of bacterial inner-membrane proteins
Abstract
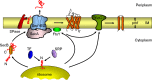
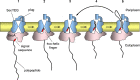
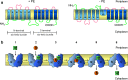
All cells must traffic proteins into and across their membranes. In bacteria, several pathways have evolved to enable protein transfer across the inner membrane, the periplasm, and the outer membrane. The major route of protein translocation in and across the cytoplasmic membrane is the general secretion pathway (Sec-pathway). The biogenesis of membrane proteins not only requires protein translocation but also coordinated targeting to the membrane beforehand and folding and assembly into their protein complexes afterwards to function properly in the cell. All these processes are responsible for the biogenesis of membrane proteins that mediate essential functions of the cell such as selective transport, energy conversion, cell division, extracellular signal sensing, and motility. This review will highlight the most recent developments on the structure and function of bacterial membrane proteins, focusing on the journey that integral membrane proteins take to find their final destination in the inner membrane.
Figures
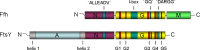
Similar articles
-
Biogenesis of β-barrel integral proteins of bacterial outer membrane.Biochemistry (Mosc). 2012 Nov;77(11):1221-36. doi: 10.1134/S0006297912110016. Biochemistry (Mosc). 2012. PMID: 23240560 Review.
-
Evidence for phospholipid export from the bacterial inner membrane by the Mla ABC transport system.Nat Microbiol. 2019 Oct;4(10):1692-1705. doi: 10.1038/s41564-019-0481-y. Epub 2019 Jun 24. Nat Microbiol. 2019. PMID: 31235958
-
Lipopolysaccharide transport to the cell surface: biosynthesis and extraction from the inner membrane.Philos Trans R Soc Lond B Biol Sci. 2015 Oct 5;370(1679):20150029. doi: 10.1098/rstb.2015.0029. Philos Trans R Soc Lond B Biol Sci. 2015. PMID: 26370941 Free PMC article. Review.
-
Protein secretion in the absence of ATP: the autotransporter, two-partner secretion and chaperone/usher pathways of gram-negative bacteria (review).Mol Membr Biol. 2005 Jan-Apr;22(1-2):63-72. doi: 10.1080/09687860500063290. Mol Membr Biol. 2005. PMID: 16092525 Review.
-
Autotransporter secretion: varying on a theme.Res Microbiol. 2013 Jul-Aug;164(6):562-82. doi: 10.1016/j.resmic.2013.03.010. Epub 2013 Apr 6. Res Microbiol. 2013. PMID: 23567321 Review.
Cited by
-
Unwrapping bacteria.PLoS Genet. 2014 Jan;10(1):e1004054. doi: 10.1371/journal.pgen.1004054. Epub 2014 Jan 2. PLoS Genet. 2014. PMID: 24391518 Free PMC article. No abstract available.
-
Dynamic disulfide scanning of the membrane-inserting Pf3 coat protein reveals multiple YidC substrate contacts.J Biol Chem. 2012 Feb 3;287(6):3769-76. doi: 10.1074/jbc.M111.307223. Epub 2011 Dec 16. J Biol Chem. 2012. PMID: 22179606 Free PMC article.
-
Domain swapping oligomerization of thermostable c-type cytochrome in E. coli cells.Sci Rep. 2016 Feb 3;6:19334. doi: 10.1038/srep19334. Sci Rep. 2016. PMID: 26838805 Free PMC article.
-
A membrane-bound esterase PA2949 from Pseudomonas aeruginosa is expressed and purified from Escherichia coli.FEBS Open Bio. 2016 Apr 19;6(5):484-93. doi: 10.1002/2211-5463.12061. eCollection 2016 May. FEBS Open Bio. 2016. PMID: 27419054 Free PMC article.
-
Effect of Ultrasound Combined with Plasma-Activated Water on Lethal and Sublethal Injury Against Escherichia coli.Foods. 2025 Apr 23;14(9):1457. doi: 10.3390/foods14091457. Foods. 2025. PMID: 40361540 Free PMC article.
References
-
- Kramer G, Rauch T, Rist W, Vorderwülbecke S, Patzelt H, Schulze-Specking A, Ban N, Deuerling E, Bukau B. L23 protein functions as a chaperone docking site on the ribosome. Nature. 2002;419:171–174. - PubMed
-
- Kaiser CM, Chang HC, Agashe VR, Lakshmipathy SK, Etchells SA, Hayer-Hartl M, Hartl FU, Barral JM. Real-time observation of trigger factor function on translating ribosomes. Nature. 2006;444:455–460. - PubMed
-
- Rutkowska A, Mayer MP, Hoffmann A, Merz F, Zachmann-Brand B, Schaffitzel C, Ban N, Deuerling E, Bukau B. Dynamics of trigger factor interaction with translating ribosomes. J Biol Chem. 2008;283:4124–4132. - PubMed
-
- Ferbitz L, Maier T, Patzelt H, Bukau B, Deuerling E, Ban N. Trigger factor in complex with the ribosome forms a molecular cradle for nascent proteins. Nature. 2004;431:590–596. - PubMed
-
- Bukau B, Deuerling E, Pfund C, Craig EA. Getting newly synthesized proteins into shape. Cell. 2000;101:119–122. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

