Fluid flow through a high cell density fluidized-bed during centrifugal bioreactor culture
- PMID: 20205172
- PMCID: PMC2997645
- DOI: 10.1002/btpr.395
Fluid flow through a high cell density fluidized-bed during centrifugal bioreactor culture
Abstract
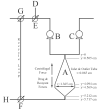
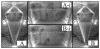
An increasing demand for products such as tissues, proteins, and antibodies from mammalian cell suspension cultures is driving interest in increasing production through high-cell density bioreactors. The centrifugal bioreactor (CCBR) retains cells by balancing settling forces with surface drag forces due to medium throughput and is capable of maintaining cell densities above 10(8) cells/mL. This article builds on a previous study where the fluid mechanics of an empty CCBR were investigated showing fluid flow is nonuniform and dominated by Coriolis forces, raising concerns about nutrient and cell distribution. In this article, we demonstrate that the previously reported Coriolis forces are still present in the CCBR, but masked by the presence of cells. Experimental dye injection observations during culture of 15 microm hybridoma cells show a continual uniform darkening of the cell bed, indicating the region of the reactor containing cells is well mixed. Simulation results also indicate the cell bed is well mixed during culture of mammalian cells ranging in size from 10 to 20 microm. However, simulations also allow for a slight concentration gradient to be identified and attributed to Coriolis forces. Experimental results show cell density increases from 0.16 to 0.26 when centrifugal force is doubled by increasing RPM from 650 to 920 at a constant inlet velocity of 6.5 cm/s; an effect also observed in the simulation. Results presented in this article indicate cells maintained in the CCBR behave as a high-density fluidized bed of cells providing a homogeneous environment to ensure optimal growth conditions.
(c) 2010 American Institute of Chemical Engineers
Figures






Similar articles
-
Trends in Monoclonal Antibody Production Using Various Bioreactor Syst.J Microbiol Biotechnol. 2021 Mar 28;31(3):349-357. doi: 10.4014/jmb.1911.11066. J Microbiol Biotechnol. 2021. PMID: 32238761 Free PMC article. Review.
-
A study of the Coriolis effect on the fluid flow profile in a centrifugal bioreactor.Biotechnol Prog. 2009 Jul-Aug;25(4):1025-34. doi: 10.1002/btpr.183. Biotechnol Prog. 2009. PMID: 19455639 Free PMC article.
-
Kinetic simulation of a centrifugal bioreactor for high population density hybridoma culture.Biotechnol Prog. 2009 Nov-Dec;25(6):1650-9. doi: 10.1002/btpr.240. Biotechnol Prog. 2009. PMID: 19806634 Free PMC article.
-
A novel continuous centrifugal bioreactor for high-density cultivation of mammalian and microbial cells.Biotechnol Bioeng. 1991 Dec 5;38(10):1190-202. doi: 10.1002/bit.260381011. Biotechnol Bioeng. 1991. PMID: 18600715
-
A fibrous-bed bioreactor for continuous production of monoclonal antibody by hybridoma.Adv Biochem Eng Biotechnol. 2004;87:61-96. doi: 10.1007/b94364. Adv Biochem Eng Biotechnol. 2004. PMID: 15217104 Review.
Cited by
-
Use of a centrifugal bioreactor for cartilaginous tissue formation from isolated chondrocytes.Biotechnol Prog. 2011 Mar-Apr;27(2):451-9. doi: 10.1002/btpr.551. Epub 2011 Feb 2. Biotechnol Prog. 2011. PMID: 21290617 Free PMC article.
-
Trends in Monoclonal Antibody Production Using Various Bioreactor Syst.J Microbiol Biotechnol. 2021 Mar 28;31(3):349-357. doi: 10.4014/jmb.1911.11066. J Microbiol Biotechnol. 2021. PMID: 32238761 Free PMC article. Review.
-
Bioengineering Outlook on Cultivated Meat Production.Micromachines (Basel). 2022 Feb 28;13(3):402. doi: 10.3390/mi13030402. Micromachines (Basel). 2022. PMID: 35334693 Free PMC article. Review.
References
-
- Landry Y, Gies JP. Drugs and their molecular targets: an updated overview. Fundam Clin Pharmacol. 2008;22:1–18. - PubMed
-
- Migliore C, Giordano S. Molecular cancer therapy: can our expectation be MET? Eur J Cancer. 2008;44:641–651. - PubMed
-
- Kundu PK, Prasad NS, Electricwala SE, Varma R, Datta D. Getting higher yields of monoclonal antibody in culture. Indian J Physiol Pharmacol. 1998;42:155–171. - PubMed
-
- Werner RG. Economic aspects of commercial manufacture of biopharmaceuticals. J Biotechnol. 2004;113:171–182. - PubMed
-
- Tang YJ, Ohashi R, Hamel JF. Perfusion culture of hybridoma cells for hyperproduction of IgG(2a) monoclonal antibody in a wave bioreactor-perfusion culture system. Biotechnol Prog. 2007;23:255–264. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

