Fluorescent analogs of biomolecular building blocks: design, properties, and applications
- PMID: 20205430
- PMCID: PMC2868948
- DOI: 10.1021/cr900301e
Fluorescent analogs of biomolecular building blocks: design, properties, and applications
Figures
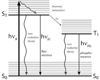


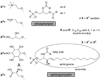
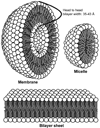
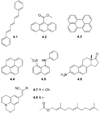
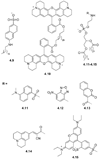
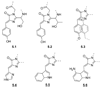
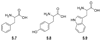
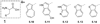
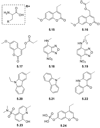
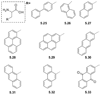
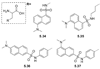
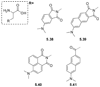
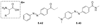
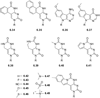
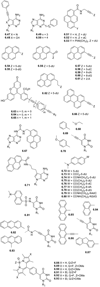
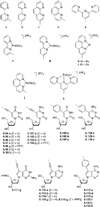
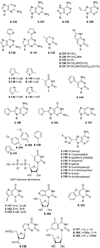
References
-
- Sameiro M, Goncalves T. Chem. Rev. 2009;109:190. - PubMed
-
- Lakowicz JR. Principles of Fluorescence Spectroscopy. 3rd ed. New York: Springer; 2006.
-
- Bacia K, Schwille P. Methods. 2003;29:74. - PubMed
-
- Valeur B. Molecular Fluorescence, Principles and Applications. Weinheim: Wiley-VCH Verlag GmbH; 2002.
-
- Turro NJ. Modern Molecular Photochemistry of Organic Molecules. New York: University Science Books; 2009.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

