Genetic analysis of the role of tumor necrosis factor receptors in functional outcome after traumatic brain injury in mice
- PMID: 20205514
- PMCID: PMC2943499
- DOI: 10.1089/neu.2009.1229
Genetic analysis of the role of tumor necrosis factor receptors in functional outcome after traumatic brain injury in mice
Abstract
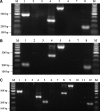
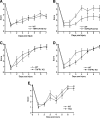
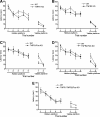
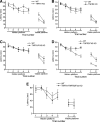
We previously reported that tumor necrosis factor-alpha (TNF-alpha) and Fas receptor induce acute cellular injury, tissue damage, and motor and cognitive deficits after controlled cortical impact (CCI) in mice (Bermpohl et al. 2007 ); however, the TNF receptors (TNFR) involved are unknown. Using a CCI model and novel mutant mice deficient in TNFR1/Fas, TNFR2/Fas, or TNFR1/TNFR2/Fas, we tested the hypothesis that the combination of TNFR2/Fas is protective, whereas TNFR1/Fas is detrimental after CCI. Uninjured knockout (KO) mice showed no differences in baseline physiological variables or motor or cognitive function. Following CCI, mice deficient in TNFR2/Fas had worse post-injury motor and Morris water maze (MWM) performance than wild-type (WT) mice (p < 0.05 group effect for wire grip score and MWM performance by repeated measures ANOVA). No differences in motor or cognitive outcome were observed in TNFR1/Fas KO, or in TNFR2 or TNFR1 single KO mice, versus WT mice. Additionally, no differences in propidium iodide (PI)-positive cells (at 6 h) or lesion size (at 14 days) were observed between WT and TNFR1/Fas or TNFR2/Fas KO mice. Somewhat surprisingly, mice deficient in TNFR1/TNFR2/Fas also had PI-positive cells, lesion size, and motor and MWM deficits similar to those of WT mice. These data suggest a protective role for TNFR2/Fas in the pathogenesis of TBI. Further studies are needed to determine whether direct or indirect effects of TNFR1 deletion in TNFR2/Fas KO mice mediate improved functional outcome in TNFR1/TNFR2/Fas KO mice after CCI.
Figures
Similar articles
-
Differential role of tumor necrosis factor receptors in mouse brain inflammatory responses in cryolesion brain injury.J Neurosci Res. 2005 Dec 1;82(5):701-16. doi: 10.1002/jnr.20680. J Neurosci Res. 2005. PMID: 16267827
-
TNF alpha and Fas mediate tissue damage and functional outcome after traumatic brain injury in mice.J Cereb Blood Flow Metab. 2007 Nov;27(11):1806-18. doi: 10.1038/sj.jcbfm.9600487. Epub 2007 Apr 4. J Cereb Blood Flow Metab. 2007. PMID: 17406655
-
Diverging mechanisms for TNF-alpha receptors in normal mouse brains and in functional recovery after injury: From gene to behavior.J Neurosci Res. 2007 Sep;85(12):2668-85. doi: 10.1002/jnr.21126. J Neurosci Res. 2007. PMID: 17131423
-
Effects of tumor necrosis factor type 1 and 2 receptor deficiencies on anorexia, growth and IgA dysregulation in mice exposed to the trichothecene vomitoxin.Food Chem Toxicol. 2002 Nov;40(11):1623-31. doi: 10.1016/s0278-6915(02)00153-9. Food Chem Toxicol. 2002. PMID: 12176089
-
TNF receptors in the microvascular pathology of acute respiratory distress syndrome and cerebral malaria.J Leukoc Biol. 1997 May;61(5):551-8. doi: 10.1002/jlb.61.5.551. J Leukoc Biol. 1997. PMID: 9129203 Review.
Cited by
-
Tumor necrosis factor in traumatic brain injury: effects of genetic deletion of p55 or p75 receptor.J Cereb Blood Flow Metab. 2013 Aug;33(8):1182-9. doi: 10.1038/jcbfm.2013.65. Epub 2013 Apr 24. J Cereb Blood Flow Metab. 2013. PMID: 23611870 Free PMC article.
-
Revisiting the critical roles of reactive microglia in traumatic brain injury.Int J Surg. 2025 Jun 1;111(6):3942-3978. doi: 10.1097/JS9.0000000000002420. Epub 2025 May 12. Int J Surg. 2025. PMID: 40358653 Free PMC article. Review.
-
Long-term upregulation of inflammation and suppression of cell proliferation in the brain of adult rats exposed to traumatic brain injury using the controlled cortical impact model.PLoS One. 2013;8(1):e53376. doi: 10.1371/journal.pone.0053376. Epub 2013 Jan 3. PLoS One. 2013. PMID: 23301065 Free PMC article.
-
Emerging Roles for the Immune System in Traumatic Brain Injury.Front Immunol. 2016 Dec 5;7:556. doi: 10.3389/fimmu.2016.00556. eCollection 2016. Front Immunol. 2016. PMID: 27994591 Free PMC article. Review.
-
Sex and Genotype Affect Mouse Hippocampal Gene Expression in Response to Blast-Induced Traumatic Brain Injury.Mol Neurobiol. 2025 Aug;62(8):9980-10005. doi: 10.1007/s12035-025-04879-5. Epub 2025 Apr 3. Mol Neurobiol. 2025. PMID: 40178780 Free PMC article.
References
-
- Beg A.A. Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274:782–784. - PubMed
-
- Bermpohl D. You Z. Korsmeyer S.J. Moskowitz M.A. Whalen M.J. Traumatic brain injury in mice deficient in Bid: effects on histopathology and functional outcome. J. Cereb. Blood Flow Metab. 2006;26:625–633. - PubMed
-
- Bermpohl D. You Z. Lo E.H. Kim H.H. abd Whalen M.J. TNF alpha and Fas mediate tissue damage and functional outcome after traumatic brain injury in mice. J. Cereb. Blood Flow Metab. 2007;27:1806–1818. - PubMed
-
- Bruce A.J. Boling W. Kindy M.S. Peschon J. Kraemer P.J. Carpenter M.K. Holtsberg F.W. Mattson M.P. Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF receptors. Nat. Med. 1996;2:788–794. - PubMed
-
- Bruce-Keller A.J. Geddes J.W. Knapp P.E. McFall R.W. Keller J.N. Holtsberg F.W. Parthasarathy S. Steiner S.M. Mattson M.P. Anti-death properties of TNF against metabolic poisoning: mitochondrial stabilization by MnSOD. J. Neuroimmunol. 1999;93:53–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

