Minocycline attenuates HIV infection and reactivation by suppressing cellular activation in human CD4+ T cells
- PMID: 20205570
- PMCID: PMC3739045
- DOI: 10.1086/651277
Minocycline attenuates HIV infection and reactivation by suppressing cellular activation in human CD4+ T cells
Abstract
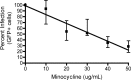
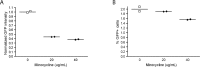
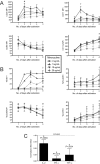
Treatment of human immunodeficiency virus (HIV) infection with highly active antiretroviral therapy (HAART) is effective but can be associated with toxic effects and is expensive. Other options may be useful for long-term therapy. The immunomodulatory antibiotic minocycline could be an effective, low-cost adjunctive treatment to HAART. Minocycline mediated a dose-dependent decrease in single-cycle CXCR4-tropic HIV infection and decreased viral RNA after infection of CD4+ T cells with HIV NL4-3. Reactivation from latency was also decreased in a primary CD4+ T cell-derived model and in resting CD4+ T cells from HIV-infected patients. Minocycline treatment resulted in significant changes in activation marker expression and inhibited proliferation and cytokine secretion of CD4+ T cells in response to activation. This study demonstrates that minocycline reduces HIV replication and reactivation and decreases CD4+ T cell activation. The anti-HIV effects of minocycline are mediated by altering the cellular environment rather than directly targeting virus, placing minocycline in the class of anticellular anti-HIV drugs.
Figures


Comment in
-
A novel use for an old drug: the potential for minocycline as anti-HIV adjuvant therapy.J Infect Dis. 2010 Apr 15;201(8):1115-7. doi: 10.1086/651278. J Infect Dis. 2010. PMID: 20205572 No abstract available.
Similar articles
-
Can HIV infection be eradicated through use of potent antiviral agents?Curr Opin Infect Dis. 2010 Dec;23(6):628-32. doi: 10.1097/QCO.0b013e32833ff1d0. Curr Opin Infect Dis. 2010. PMID: 20847693 Review.
-
Neuroprotective and anti-human immunodeficiency virus activity of minocycline.JAMA. 2005 Apr 27;293(16):2003-11. doi: 10.1001/jama.293.16.2003. JAMA. 2005. PMID: 15855434
-
The effect of intensification with raltegravir on the HIV-1 reservoir of latently infected memory CD4 T cells in suppressed patients.AIDS. 2012 Sep 24;26(15):1885-94. doi: 10.1097/QAD.0b013e3283584521. AIDS. 2012. PMID: 22992577 Clinical Trial.
-
[Viral dynamics in the course of HIV-1 infection: pathogenetic features and new therapeutic prospects].Pathologica. 2000 Aug;92(4):291-3. Pathologica. 2000. PMID: 11029891 Italian. No abstract available.
-
The challenge of finding a cure for HIV infection.Science. 2009 Mar 6;323(5919):1304-7. doi: 10.1126/science.1165706. Science. 2009. PMID: 19265012 Review.
Cited by
-
Minocycline: far beyond an antibiotic.Br J Pharmacol. 2013 May;169(2):337-52. doi: 10.1111/bph.12139. Br J Pharmacol. 2013. PMID: 23441623 Free PMC article. Review.
-
Attenuation of pathogenic immune responses during infection with human and simian immunodeficiency virus (HIV/SIV) by the tetracycline derivative minocycline.PLoS One. 2014 Apr 14;9(4):e94375. doi: 10.1371/journal.pone.0094375. eCollection 2014. PLoS One. 2014. PMID: 24732038 Free PMC article.
-
Antiviral Activity of Approved Antibacterial, Antifungal, Antiprotozoal and Anthelmintic Drugs: Chances for Drug Repurposing for Antiviral Drug Discovery.J Exp Pharmacol. 2022 Mar 8;14:97-115. doi: 10.2147/JEP.S346006. eCollection 2022. J Exp Pharmacol. 2022. PMID: 35299994 Free PMC article. Review.
-
Drug repurposing for new, efficient, broad spectrum antivirals.Virus Res. 2019 Apr 15;264:22-31. doi: 10.1016/j.virusres.2019.02.011. Epub 2019 Feb 19. Virus Res. 2019. PMID: 30794895 Free PMC article. Review.
-
HIV reservoirs and strategies for eradication.Curr HIV/AIDS Rep. 2012 Mar;9(1):5-15. doi: 10.1007/s11904-011-0108-2. Curr HIV/AIDS Rep. 2012. PMID: 22249405 Review.
References
-
- d'Arminio Monforte A, Lepri AC, Rezza G, et al. Insights into the reasons for discontinuation of the first highly active antiretroviral therapy (HAART) regimen in a cohort of antiretroviral nai"ve patients I.CO.N.A Study Group Italian Cohort of Antiretroviral-Naï ve Patients. AIDS. 2000;14:499–507. - PubMed
-
- Salama NN, Endsley A, Ho RJ. Recent developments in drug targets and delivery of anti-HIV drugs. Infect Disord Drug Targets. 2006;6:107–119. - PubMed
-
- Shehu-Xhilaga M, Tachedjian G, Crowe SM, Kedzierska K. Antiretroviral compounds: mechanisms underlying failure of HAART to eradicate HIV-1. Curr Med Chem. 2005;12:1705–1719. - PubMed
-
- McGee B, Smith N, Aweeka F. HIV pharmacology: barriers to the eradication of HIV from the CNS. HIV Clin Trials. 2006;7:142–153. - PubMed
-
- Saivin S, Houin G. Clinical pharmacokinetics of doxycycline and minocycline. Clin Pharmacokinet. 1988;15:355–366. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

