DREAM (downstream regulatory element antagonist modulator) contributes to synaptic depression and contextual fear memory
- PMID: 20205763
- PMCID: PMC2822766
- DOI: 10.1186/1756-6606-3-3
DREAM (downstream regulatory element antagonist modulator) contributes to synaptic depression and contextual fear memory
Abstract
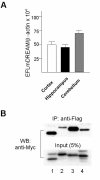
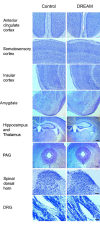
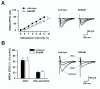
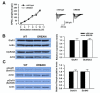
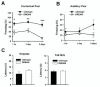
The downstream regulatory element antagonist modulator (DREAM), a multifunctional Ca2+-binding protein, binds specifically to DNA and several nucleoproteins regulating gene expression and with proteins outside the nucleus to regulate membrane excitability or calcium homeostasis. DREAM is highly expressed in the central nervous system including the hippocampus and cortex; however, the roles of DREAM in hippocampal synaptic transmission and plasticity have not been investigated. Taking advantage of transgenic mice overexpressing a Ca2+-insensitive DREAM mutant (TgDREAM), we used integrative methods including electrophysiology, biochemistry, immunostaining, and behavior tests to study the function of DREAM in synaptic transmission, long-term plasticity and fear memory in hippocampal CA1 region. We found that NMDA receptor but not AMPA receptor-mediated current was decreased in TgDREAM mice. Moreover, synaptic plasticity, such as long-term depression (LTD) but not long-term potentiation (LTP), was impaired in TgDREAM mice. Biochemical experiments found that DREAM interacts with PSD-95 and may inhibit NMDA receptor function through this interaction. Contextual fear memory was significantly impaired in TgDREAM mice. By contrast, sensory responses to noxious stimuli were not affected. Our results demonstrate that DREAM plays a novel role in postsynaptic modulation of the NMDA receptor, and contributes to synaptic plasticity and behavioral memory.
Figures



Similar articles
-
Neurabin contributes to hippocampal long-term potentiation and contextual fear memory.PLoS One. 2008 Jan 9;3(1):e1407. doi: 10.1371/journal.pone.0001407. PLoS One. 2008. PMID: 18183288 Free PMC article.
-
Defective synaptic transmission and structure in the dentate gyrus and selective fear memory impairment in the Rsk2 mutant mouse model of Coffin-Lowry syndrome.Neurobiol Dis. 2013 Oct;58:156-68. doi: 10.1016/j.nbd.2013.05.016. Epub 2013 Jun 3. Neurobiol Dis. 2013. PMID: 23742761
-
Impaired synaptic clustering of postsynaptic density proteins and altered signal transmission in hippocampal neurons, and disrupted learning behavior in PDZ1 and PDZ2 ligand binding-deficient PSD-95 knockin mice.Mol Brain. 2012 Dec 26;5:43. doi: 10.1186/1756-6606-5-43. Mol Brain. 2012. PMID: 23268962 Free PMC article.
-
[Molecular mechanisms for memory formation].Brain Nerve. 2008 Jul;60(7):707-15. Brain Nerve. 2008. PMID: 18646610 Review. Japanese.
-
Hippocampal synaptic plasticity, spatial memory and anxiety.Nat Rev Neurosci. 2014 Mar;15(3):181-92. doi: 10.1038/nrn3677. Nat Rev Neurosci. 2014. PMID: 24552786 Review.
Cited by
-
Nicotine-prevented learning and memory impairment in REM sleep-deprived rat is modulated by DREAM protein in the hippocampus.Brain Behav. 2017 Apr 20;7(6):e00704. doi: 10.1002/brb3.704. eCollection 2017 Jun. Brain Behav. 2017. PMID: 28638710 Free PMC article.
-
Understanding the physiological roles of the neuronal calcium sensor proteins.Mol Brain. 2012 Jan 23;5(1):2. doi: 10.1186/1756-6606-5-2. Mol Brain. 2012. PMID: 22269068 Free PMC article. Review.
-
Global Gene Knockout of Kcnip3 Enhances Pain Sensitivity and Exacerbates Negative Emotions in Rats.Front Mol Neurosci. 2019 Jan 25;12:5. doi: 10.3389/fnmol.2019.00005. eCollection 2019. Front Mol Neurosci. 2019. PMID: 30740043 Free PMC article.
-
Protein-Protein Interactions: Gene Acronym Redundancies and Current Limitations Precluding Automated Data Integration.Proteomes. 2013 May 31;1(1):3-24. doi: 10.3390/proteomes1010003. Proteomes. 2013. PMID: 28250396 Free PMC article.
-
Downstream Regulatory Element Antagonist Modulator (DREAM), a target for anti-thrombotic agents.Pharmacol Res. 2017 Mar;117:283-287. doi: 10.1016/j.phrs.2017.01.002. Epub 2017 Jan 5. Pharmacol Res. 2017. PMID: 28065857 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

