CD40mAb adjuvant induces a rapid antibody response that may be beneficial in post-exposure prophylaxis
- PMID: 20205811
- PMCID: PMC2824643
- DOI: 10.1186/1476-8518-8-1
CD40mAb adjuvant induces a rapid antibody response that may be beneficial in post-exposure prophylaxis
Abstract
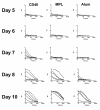
Active vaccination can be effective as a post-exposure prophylaxis, but the rapidity of the immune response induced, relative to the incubation time of the pathogen, is critical. We show here that CD40mAb conjugated to antigen induces a more rapid specific antibody response than currently used immunological adjuvants, alum and monophosphoryl lipid A.
Figures
References
-
- Inglesby TV, O'Toole T, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Friedlander AM, Gerberding J, Hauer J, Hughes J, McDade J, Osterholm MT, Parker G, Perl TM, Russell PK, Tonat K. Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA. 2002;287:2236–2252. doi: 10.1001/jama.287.17.2236. - DOI - PubMed
LinkOut - more resources
Full Text Sources

