Axotomy-induced neurotrophic withdrawal causes the loss of phenotypic differentiation and downregulation of NGF signalling, but not death of septal cholinergic neurons
- PMID: 20205865
- PMCID: PMC2826326
- DOI: 10.1186/1750-1326-5-5
Axotomy-induced neurotrophic withdrawal causes the loss of phenotypic differentiation and downregulation of NGF signalling, but not death of septal cholinergic neurons
Abstract
Background: Septal cholinergic neurons account for most of the cholinergic innervations of the hippocampus, playing a key role in the regulation of hippocampal synaptic activity. Disruption of the septo-hippocampal pathway by an experimental transection of the fimbria-fornix drastically reduces the target-derived trophic support received by cholinergic septal neurons, mainly nerve growth factor (NGF) from the hippocampus. Axotomy of cholinergic neurons induces a reduction in the number of neurons positive for cholinergic markers in the medial septum. In several studies, the reduction of cholinergic markers has been interpreted as analogous to the neurodegeneration of cholinergic cells, ruling out the possibility that neurons lose their cholinergic phenotype without dying. Understanding the mechanism of cholinergic neurodegeneration after axotomy is relevant, since this paradigm has been extensively explored as an animal model of the cholinergic impairment observed in neuropathologies such as Alzheimer's disease.The principal aim of this study was to evaluate, using modern quantitative confocal microscopy, neurodegenerative changes in septal cholinergic neurons after axotomy and to assess their response to delayed infusion of NGF in rats.
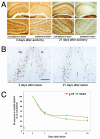
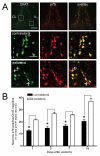
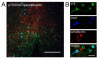
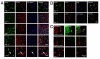
Results: We found that there is a slow reduction of cholinergic cells labeled by ChAT and p75 after axotomy. However, this phenomenon is not accompanied by neurodegenerative changes or by a decrease in total neuronal number in the medial septum. Although the remaining axotomized-neurons appear healthy, they are unable to respond to delayed NGF infusion.
Conclusions: Our results demonstrate that at 3 weeks, axotomized cholinergic neurons lose their cholinergic phenotype without dying and down-regulate their NGF-receptors, precluding the possibility of a response to NGF. Therefore, the physiological role of NGF in the adult septal cholinergic system is to support phenotypic differentiation and not survival of neurons. This evidence raises questions about the relationship between transcriptional regulation of the cholinergic phenotype by retrograde-derived trophic signaling and the transcriptional changes experienced when retrograde transport is impaired due to neuropathological conditions.
Figures
Similar articles
-
Nerve growth factor (NGF) reverses axotomy-induced decreases in choline acetyltransferase, NGF receptor and size of medial septum cholinergic neurons.Brain Res. 1989 Dec 25;505(1):29-38. doi: 10.1016/0006-8993(89)90112-1. Brain Res. 1989. PMID: 2558781
-
Mouse nerve growth factor prevents degeneration of axotomized basal forebrain cholinergic neurons in the monkey.J Neurosci. 1990 Dec;10(12):3801-13. doi: 10.1523/JNEUROSCI.10-12-03801.1990. J Neurosci. 1990. PMID: 2269884 Free PMC article.
-
BMP9 protects septal neurons from axotomy-evoked loss of cholinergic phenotype.PLoS One. 2011;6(6):e21166. doi: 10.1371/journal.pone.0021166. Epub 2011 Jun 13. PLoS One. 2011. PMID: 21695154 Free PMC article.
-
Role of NGF in axotomy-induced c-Jun expression in medial septal cholinergic neurons.Int J Dev Neurosci. 1998 Nov-Dec;16(7-8):691-703. doi: 10.1016/s0736-5748(98)00079-3. Int J Dev Neurosci. 1998. PMID: 10198817 Review.
-
Trophic and growth-regulating mechanisms in the central nervous system monitored by intracerebral neural transplants.Ciba Found Symp. 1987;126:143-59. doi: 10.1002/9780470513422.ch9. Ciba Found Symp. 1987. PMID: 3556083 Review.
Cited by
-
Chemogenetic Inactivation of Dorsal Anterior Cingulate Cortex Neurons Disrupts Attentional Behavior in Mouse.Neuropsychopharmacology. 2016 Mar;41(4):1014-23. doi: 10.1038/npp.2015.229. Epub 2015 Jul 30. Neuropsychopharmacology. 2016. PMID: 26224620 Free PMC article.
-
Adolescent binge ethanol-induced loss of basal forebrain cholinergic neurons and neuroimmune activation are prevented by exercise and indomethacin.PLoS One. 2018 Oct 8;13(10):e0204500. doi: 10.1371/journal.pone.0204500. eCollection 2018. PLoS One. 2018. PMID: 30296276 Free PMC article.
-
Exercise leads to the re-emergence of the cholinergic/nestin neuronal phenotype within the medial septum/diagonal band and subsequent rescue of both hippocampal ACh efflux and spatial behavior.Exp Neurol. 2016 Apr;278:62-75. doi: 10.1016/j.expneurol.2016.01.018. Epub 2016 Jan 30. Exp Neurol. 2016. PMID: 26836322 Free PMC article.
-
Dysregulation of neurotrophin expression in prefrontal cortex and nucleus basalis magnocellularis during and after adolescent intermittent ethanol exposure.Alcohol. 2024 Nov;120:1-14. doi: 10.1016/j.alcohol.2024.06.001. Epub 2024 Jun 17. Alcohol. 2024. PMID: 38897258
-
Reversal of Neuronal Atrophy: Role of Cellular Immunity in Neuroplasticity and Aging.J Neurol Disord. 2014 Jul;2(4):1000170. doi: 10.4172/2329-6895.1000170. J Neurol Disord. 2014. PMID: 25505790 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

