Small chloroplast-targeted DnaJ proteins are involved in optimization of photosynthetic reactions in Arabidopsis thaliana
- PMID: 20205940
- PMCID: PMC2844072
- DOI: 10.1186/1471-2229-10-43
Small chloroplast-targeted DnaJ proteins are involved in optimization of photosynthetic reactions in Arabidopsis thaliana
Abstract
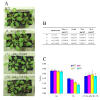
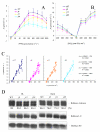
Background: DnaJ proteins participate in many metabolic pathways through dynamic interactions with various components of these processes. The role of three small chloroplast-targeted DnaJ proteins, AtJ8 (At1 g80920), AtJ11 (At4 g36040) and AtJ20 (At4 g13830), was investigated here using knock-out mutants of Arabidopsis thaliana. Photochemical efficiency, capacity of CO2 assimilation, stabilization of Photosystem (PS) II dimers and supercomplexes under high light illumination, energy distribution between PSI and PSII and phosphorylation of PSII-LHCII proteins, global gene expression profiles and oxidative stress responses of these DnaJ mutants were analyzed.
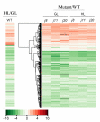
Results: Knockout of one of these proteins caused a series of events including a decrease in photosynthetic efficiency, destabilization of PSII complexes and loss of control for balancing the redox reactions in chloroplasts. Data obtained with DNA microarray analysis demonstrated that the lack of one of these DnaJ proteins triggers a global stress response and therefore confers the plants greater tolerance to oxidative stress induced by high light or methyl viologen treatments. Expression of a set of genes encoding enzymes that detoxify reactive oxygen species (ROS) as well as a number of stress-related transcription factors behaved in the mutants at growth light similarly to that when wild-type (WT) plants were transferred to high light. Also a set of genes related to redox regulation were upregulated in the mutants. On the other hand, although the three DnaJ proteins reside in chloroplasts, the expression of most genes encoding thylakoid membrane proteins was not changed in the mutants.
Conclusion: It is proposed that the tolerance of the DnaJ protein knockout plants to oxidative stress occurs at the expense of the flexibility of photosynthetic reactions. Despite the fact that the effects of the individual protein knockout on the response of plants to high light treatment are quite similar, it is conceivable that both specific- and cross-talk functions exist between the three small chloroplast-targeted DnaJ proteins, AtJ8, AtJ11 and AtJ20.
Figures





References
-
- Voos W, Rottgers K. Molecular chaperones as essential mediators of mitochondria biogenesis. Biochim Biophys Acta. 2002;1592:52–62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

