Retinal ultrastructure of murine models of dry age-related macular degeneration (AMD)
- PMID: 20206286
- PMCID: PMC2854213
- DOI: 10.1016/j.preteyeres.2010.02.002
Retinal ultrastructure of murine models of dry age-related macular degeneration (AMD)
Abstract
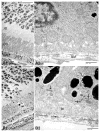
Age-related macular degeneration (AMD) is the most prevalent form of irreversible blindness worldwide in the elderly population. The pathology of dry AMD consists of macular degeneration of photoreceptors and the RPE, lipofuscin (A2E) accumulation, and drusen formation. Mice have been widely used for generating models that simulate human AMD features for investigating the pathogenesis, treatment and prevention of the disease. Although the mouse has no macula, focal atrophy of photoreceptors and RPE, lipofuscin accumulation, and increased A2E can develop in aged mouse eyes. However, drusen are rarely seen in mice because of their simpler Bruch's membrane and different process of lipofuscin extrusion compared with humans. Thus, analyzing basal deposits at the ultrastructural level and understanding the ultrastructural pathologic differences between various mouse AMD models are critical to comprehending the significance of research findings and response to possible therapeutic options for dry AMD. Based on the multifactorial pathogenesis of AMD, murine dry AMD models can be classified into three groups. First, genetically engineered mice that target genes related to juvenile macular dystrophies are the most common models, and they include abcr(-/-) (Stargardt disease), transgenic ELOVL4 (Stargardt-3 dominant inheritary disease), Efemp1(R345W/R345W) (Doyne honeycomb retinal dystrophy), and Timp3(S156C/S156C) (Sorsby fundus dystrophy) mice. Other murine models target genes relevant to AMD, including inflammatory genes such as Cfh(-/-), Ccl2(-/-), Ccr2(-/-), Cx3cr1(-/-), and Ccl2(-/-)/cx3cr1(-/-), oxidative stress associated genes such as Sod1(-/-) and Sod2 knockdown, metabolic pathway genes such as neprilysin(-/-) (amyloid beta), transgenic mcd/mcd (cathepsin D), Cp(-/-)/Heph(-/Y) (ferroxidase ceruloplasmin/hepaestin, iron metabolism), and transgenic ApoE4 on high fat and high cholesterol diet (lipid metabolism). Second, mice have also been immunologically manipulated by immunization with carboxyethylpyrrole (CEP), an oxidative fragment of DHA found in drusen, and found to present with dry AMD features. Third, natural mouse strains such as arrd2/arrd2 (Mdm gene mutation) and the senescence accelerated mice (SAM) spontaneously develop features of dry AMD like photoreceptor atrophy and thickening of Bruch's membrane. All the aforementioned models develop retinal lesions with various features that simulate dry AMD lesions: focal photoreceptor degeneration, abnormal RPE with increased lipofuscin, basal infolding, decreased melanosomes and degeneration. However, Bruch's membrane changes are less common. Most mice develop retinal lesions at an older age (6-24 months, depending on the models), while the Ccl2(-/-)/cx3cr1(-/-) mice develop lesions by 4-6 weeks. Although murine models present various degrees of retinal and/or RPE degeneration, classical drusen is extremely rare. Using electron microscopy, small drusenoid deposits are found between RPE and Bruch's membrane in a few models including Efemp1(R345W/R345W), Ccl2(-/-)/cx3cr1(-/-), neprilysin(-/-), transgenic mcd/mcd, and ApoE4 transgenic mice on a high fat diet. High A2E levels are measured in the retinas of abcr(-/-), transgenic ELOVL4, and Ccl2(-/-)/cx3cr1(-/-) mice. In summary, murine models provide useful tools for studying AMD pathogenesis and evaluating novel therapies for this disease. This review compares the major dry AMD murine models and discusses retinal pathology at the ultrastructural level.
Published by Elsevier Ltd.
Figures


Similar articles
-
The R345W mutation in EFEMP1 is pathogenic and causes AMD-like deposits in mice.Hum Mol Genet. 2007 Oct 15;16(20):2411-22. doi: 10.1093/hmg/ddm198. Epub 2007 Jul 30. Hum Mol Genet. 2007. PMID: 17666404
-
Murine ccl2/cx3cr1 deficiency results in retinal lesions mimicking human age-related macular degeneration.Invest Ophthalmol Vis Sci. 2007 Aug;48(8):3827-36. doi: 10.1167/iovs.07-0051. Invest Ophthalmol Vis Sci. 2007. PMID: 17652758 Free PMC article.
-
Dry age-related macular degeneration like pathology in aged 5XFAD mice: Ultrastructure and microarray analysis.Oncotarget. 2017 Jun 20;8(25):40006-40018. doi: 10.18632/oncotarget.16967. Oncotarget. 2017. PMID: 28467791 Free PMC article.
-
Sorsby fundus dystrophy - A review of pathology and disease mechanisms.Exp Eye Res. 2017 Dec;165:35-46. doi: 10.1016/j.exer.2017.08.014. Epub 2017 Aug 26. Exp Eye Res. 2017. PMID: 28847738 Review.
-
Drusen in age-related macular degeneration: pathogenesis, natural course, and laser photocoagulation-induced regression.Surv Ophthalmol. 1999 Jul-Aug;44(1):1-29. doi: 10.1016/s0039-6257(99)00072-7. Surv Ophthalmol. 1999. PMID: 10466585 Review.
Cited by
-
Transgenic Mice Overexpressing Serum Retinol-Binding Protein Develop Progressive Retinal Degeneration through a Retinoid-Independent Mechanism.Mol Cell Biol. 2015 Aug;35(16):2771-89. doi: 10.1128/MCB.00181-15. Epub 2015 Jun 8. Mol Cell Biol. 2015. PMID: 26055327 Free PMC article.
-
Nutrient supplementation with n3 polyunsaturated fatty acids, lutein, and zeaxanthin decrease A2E accumulation and VEGF expression in the retinas of Ccl2/Cx3cr1-deficient mice on Crb1rd8 background.J Nutr. 2013 Jul;143(7):1129-35. doi: 10.3945/jn.112.169649. Epub 2013 May 15. J Nutr. 2013. PMID: 23677863 Free PMC article.
-
Sub-Retinal Injection of Human Lipofuscin in the Mouse - A Model of "Dry" Age-Related Macular Degeneration?Aging Dis. 2023 Feb 1;14(1):184-203. doi: 10.14336/AD.2022.0626. eCollection 2023 Feb 1. Aging Dis. 2023. PMID: 36818570 Free PMC article.
-
Orally active multi-functional antioxidants are neuroprotective in a rat model of light-induced retinal damage.PLoS One. 2011;6(7):e21926. doi: 10.1371/journal.pone.0021926. Epub 2011 Jul 14. PLoS One. 2011. PMID: 21779355 Free PMC article.
-
Cellular and 3D optical coherence tomography assessment during the initiation and progression of retinal degeneration in the Ccl2/Cx3cr1-deficient mouse.Exp Eye Res. 2011 Nov;93(5):636-48. doi: 10.1016/j.exer.2011.07.017. Epub 2011 Aug 16. Exp Eye Res. 2011. PMID: 21854772 Free PMC article.
References
-
- Akhmedov NB, Piriev NI, Chang B, Rapoport AL, Hawes NL, Nishina PM, Nusinowitz S, Heckenlively JR, Roderick TH, Kozak CA, Danciger M, Davisson MT, Farber DB. A deletion in a photoreceptor-specific nuclear receptor mRNA causes retinal degeneration in the rd7 mouse. Proc Natl Acad Sci U S A. 2000;97:5551–6. - PMC - PubMed
-
- Allikmets R, Singh N, Sun H, Shroyer NF, Hutchinson A, Chidambaram A, Gerrard B, Baird L, Stauffer D, Peiffer A. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet. 1997;15:236–46. - PubMed
-
- Ambasudhan R, Wang X, Jablonski MM, Thompson DA, Lagali PS, Wong PW, Sieving PA, Ayyagari R. Atrophic macular degeneration mutations in ELOVL4 result in the intracellular misrouting of the protein. Genomics. 2004;83:615–25. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

