Meeting the challenges of on-host and off-host water balance in blood-feeding arthropods
- PMID: 20206630
- PMCID: PMC2918697
- DOI: 10.1016/j.jinsphys.2010.02.014
Meeting the challenges of on-host and off-host water balance in blood-feeding arthropods
Abstract
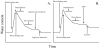
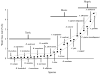
In this review, we describe water balance requirements of blood-feeding arthropods, particularly contrasting dehydration tolerance during the unfed, off-host state and the challenges of excess water that accompany receipt of the bloodmeal. Most basic water balance characteristics during the off-host stage are applicable to other terrestrial arthropods, as well. A well-coordinated suite of responses enable arthropods to conserve water resources, enhance their desiccation tolerance, and increase their water supplies by employing a diverse array of molecular, structural and behavioral responses. Water loss rates during the off-host phase are particularly useful for generating a scheme to classify vectors according to their habitat requirements for water, thus providing a convenient tool with potential predictive power for defining suitable current and future vector habitats. Blood-feeding elicits an entirely different set of challenges as the vector responds to overhydration by quickly increasing its rate of cuticular water loss and elevating the rate of diuresis to void excess water and condense the bloodmeal. Immature stages that feed on blood normally have a net increase in water content at the end of a blood-feeding cycle, but in adults the water content reverts to the pre-feeding level when the cycle is completed. Common themes are evident in diverse arthropods that feed on blood, particularly the physiological mechanisms used to respond to the sudden influx of water as well as the mechanisms used to counter water shortfalls that are encountered during the non-feeding, off-host state.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
[Specificity of host-parasite relations between arthropods and terrestrial vertebrates].Parazitologiia. 2001 Nov-Dec;35(6):473-89. Parazitologiia. 2001. PMID: 11881133 Review. Russian.
-
Pathogen transmission in relation to feeding and digestion by haematophagous Arthropods.Acta Trop. 1975;32(2):116-24. Acta Trop. 1975. PMID: 240257
-
Desiccation tolerance and drought acclimation in the Antarctic collembolan Cryptopygus antarcticus.J Insect Physiol. 2008 Oct-Nov;54(10-11):1432-9. doi: 10.1016/j.jinsphys.2008.08.004. Epub 2008 Aug 7. J Insect Physiol. 2008. PMID: 18761345
-
Sleep: An Essential and Understudied Process in the Biology of Blood-Feeding Arthropods.Integr Comp Biol. 2023 Sep 15;63(3):530-547. doi: 10.1093/icb/icad097. Integr Comp Biol. 2023. PMID: 37429615 Free PMC article. Review.
-
Dehydration Dynamics in Terrestrial Arthropods: From Water Sensing to Trophic Interactions.Annu Rev Entomol. 2023 Jan 23;68:129-149. doi: 10.1146/annurev-ento-120120-091609. Epub 2022 Oct 21. Annu Rev Entomol. 2023. PMID: 36270273 Free PMC article. Review.
Cited by
-
Cloning and functional characterization of inward-rectifying potassium (Kir) channels from Malpighian tubules of the mosquito Aedes aegypti.Insect Biochem Mol Biol. 2013 Jan;43(1):75-90. doi: 10.1016/j.ibmb.2012.09.009. Epub 2012 Oct 17. Insect Biochem Mol Biol. 2013. PMID: 23085358 Free PMC article.
-
Predicting climate-driven distribution shifts in Hyalomma marginatum (Ixodidae).Parasitology. 2023 Sep;150(10):883-893. doi: 10.1017/S0031182023000689. Epub 2023 Jul 31. Parasitology. 2023. PMID: 37519234 Free PMC article.
-
Emerging roles of aquaporins in relation to the physiology of blood-feeding arthropods.J Comp Physiol B. 2014 Oct;184(7):811-25. doi: 10.1007/s00360-014-0836-x. Epub 2014 Jun 19. J Comp Physiol B. 2014. PMID: 24942313 Review.
-
Stress Tolerance of Bed Bugs: A Review of Factors That Cause Trauma to Cimex lectularius and C. Hemipterus.Insects. 2011 Apr 29;2(2):151-72. doi: 10.3390/insects2020151. Insects. 2011. PMID: 26467619 Free PMC article. Review.
-
The interplay between temperature, Trypanosoma cruzi parasite load, and nutrition: Their effects on the development and life-cycle of the Chagas disease vector Rhodnius prolixus.PLoS Negl Trop Dis. 2024 Feb 2;18(2):e0011937. doi: 10.1371/journal.pntd.0011937. eCollection 2024 Feb. PLoS Negl Trop Dis. 2024. PMID: 38306403 Free PMC article.
References
-
- Adams TS. Hematophagy and hormone release. Annals of the Entomological Society of America. 1999;92:1–23.
-
- Akman L, Yamashita A, Watanabe H, Oshima K, Shiba T, Hattori M, Aksoy S. Genome sequence of the endocellular obligate symbiont of tsetse flies, Wigglesworthia glossinidia. Nature Genetics. 2002;32:402–407. - PubMed
-
- Aksoy S, Rio RVM. Interactions among multiple genomes: tsetse, its symbionts and trypanosomes. Insect Biochemistry and Molecular Biology. 2005;35:691–698. - PubMed
-
- Andersen SO, Roepstorff P. The extensible alloscutal cuticle of the tick, Ixodes ricinus. Insect Biochemistry and Molecular Biology. 2005;35:1181–1188. - PubMed
-
- Archer MA, Bradley TJ, Mueller LD, Rose MR. Using experimental evolution to study the physiological mechanisms of desiccation resistance in Drosophila melanogaster. Physiological Biochemistry and Zoology. 2007;80:386–398. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

