Hyporesponsiveness to re-challenge dose following pneumococcal polysaccharide vaccine at 12 months of age, a randomized controlled trial
- PMID: 20206670
- PMCID: PMC2854305
- DOI: 10.1016/j.vaccine.2010.02.087
Hyporesponsiveness to re-challenge dose following pneumococcal polysaccharide vaccine at 12 months of age, a randomized controlled trial
Abstract
Background: To evaluate the immunological impact of the 23-valent pneumococcal polysaccharide vaccine (23vPPS) at 12 months, for children who have received zero to three infant doses of seven-valent pneumococcal conjugate vaccine (PCV), on responses to a subsequent exposure to a small dose of 23vPPS (mPPS).
Methods: Five hundred and fifty-two Fijian infants were stratified by ethnicity and randomized into eight groups to receive zero, one, two, or three PCV doses at 14 weeks, six and 14 weeks, or six, ten, and 14 weeks. Within each group, half received 23vPPS at 12 months and all received mPPS at 17 months. Sera were taken prior and one month post-mPPS.
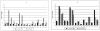
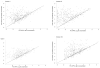
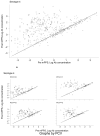
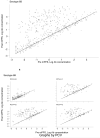
Findings: By 17 months, geometric mean antibody concentrations (GMC) to all 23 serotypes in 23vPPS were significantly higher in children who had received 23vPPS at 12 months compared to those who had not. Post-mPPS, children who had not received the 12 month 23vPPS had a significantly higher GMC for all PCV serotypes compared with those who had (each p<0.02). For the non-PCV serotypes, children who had not received the 12 month 23vPPS had significantly higher GMC for six of 16 non-PCV serotypes (7F, 9N, 12F, 19A, 22F, 33F) than those who did (each p<0.02). After adjusting for the pre-mPPS level, exposure to 23vPPS was associated with a lower response to mPPS for all serotypes (each p<0.001).
Interpretation: Despite higher antibody concentrations at 17 months in children who had received 23vPPS at 12 months, the response to a re-challenge was poor for all 23 serotypes compared to children who had not received the 12 month 23vPPS.
Trial registration: ClinicalTrials.gov NCT00170612.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Serotype-specific avidity is achieved following a single dose of the 7-valent pneumococcal conjugate vaccine, and is enhanced by 23-valent pneumococcal polysaccharide booster at 12 months.Vaccine. 2011 Jun 15;29(27):4499-506. doi: 10.1016/j.vaccine.2011.04.038. Epub 2011 May 1. Vaccine. 2011. PMID: 21539882 Free PMC article. Clinical Trial.
-
Pneumococcal nasopharyngeal carriage following reduced doses of a 7-valent pneumococcal conjugate vaccine and a 23-valent pneumococcal polysaccharide vaccine booster.Clin Vaccine Immunol. 2010 Dec;17(12):1970-6. doi: 10.1128/CVI.00117-10. Epub 2010 Oct 13. Clin Vaccine Immunol. 2010. PMID: 20943882 Free PMC article. Clinical Trial.
-
Opsonophagocytic activity following a reduced dose 7-valent pneumococcal conjugate vaccine infant primary series and 23-valent pneumococcal polysaccharide vaccine at 12 months of age.Vaccine. 2011 Jan 10;29(3):535-44. doi: 10.1016/j.vaccine.2010.10.046. Epub 2010 Oct 31. Vaccine. 2011. PMID: 21044669 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of the 23-valent pneumococcal polysaccharide vaccine at 12 months of age, following one, two, or three doses of the 7-valent pneumococcal conjugate vaccine in infancy.Vaccine. 2010 Apr 19;28(18):3086-94. doi: 10.1016/j.vaccine.2010.02.065. Epub 2010 Mar 1. Vaccine. 2010. PMID: 20199764 Free PMC article. Clinical Trial.
-
Immunogenicity following revaccination or sequential vaccination with 23-valent pneumococcal polysaccharide vaccine (PPSV23) in older adults and those at increased risk of pneumococcal disease: a review of the literature.Expert Rev Vaccines. 2021 Mar;20(3):257-267. doi: 10.1080/14760584.2021.1889374. Epub 2021 Mar 2. Expert Rev Vaccines. 2021. PMID: 33567914 Review.
Cited by
-
Reflections on pneumonia in the tropics.Pneumonia (Nathan). 2014 Dec 1;4:1-7. doi: 10.15172/pneu.2014.4/416. eCollection 2014. Pneumonia (Nathan). 2014. PMID: 31641567 Free PMC article. Review.
-
Safety and immunogenicity of 15-valent pneumococcal conjugate vaccine compared to 13-valent pneumococcal conjugate vaccine in adults ≥65 years of age previously vaccinated with 23-valent pneumococcal polysaccharide vaccine.Hum Vaccin Immunother. 2019;15(3):540-548. doi: 10.1080/21645515.2018.1532250. Epub 2018 Nov 14. Hum Vaccin Immunother. 2019. PMID: 30427749 Free PMC article. Clinical Trial.
-
Interlaboratory Comparison of the Pneumococcal Multiplex Opsonophagocytic Assays and Their Level of Agreement for Determination of Antibody Function in Pediatric Sera.mSphere. 2018 Apr 25;3(2):e00070-18. doi: 10.1128/mSphere.00070-18. Print 2018 Apr 25. mSphere. 2018. PMID: 29695620 Free PMC article.
-
Serotype-specific avidity is achieved following a single dose of the 7-valent pneumococcal conjugate vaccine, and is enhanced by 23-valent pneumococcal polysaccharide booster at 12 months.Vaccine. 2011 Jun 15;29(27):4499-506. doi: 10.1016/j.vaccine.2011.04.038. Epub 2011 May 1. Vaccine. 2011. PMID: 21539882 Free PMC article. Clinical Trial.
-
Safety and Immunogenicity of Pneumococcal Conjugate Vaccines in a High-risk Population: A Randomized Controlled Trial of 10-Valent and 13-Valent Pneumococcal Conjugate Vaccine in Papua New Guinean Infants.Clin Infect Dis. 2019 Apr 24;68(9):1472-1481. doi: 10.1093/cid/ciy743. Clin Infect Dis. 2019. PMID: 30184183 Free PMC article. Clinical Trial.
References
-
- Pneumococcal conjugate vaccine for childhood immunization--WHO position paper. Wkly Epidemiol Rec. 2007 Mar 23;82(12):93–104. - PubMed
-
- Cherian T. WHO expert consultation on serotype composition of pneumococcal conjugate vaccines for use in resource-poor developing countries, 26-27 October 2006, Geneva. Vaccine. 2007 Sep 4;25(36):6557–64. - PubMed
-
- Hausdorff WP, Bryant J, Kloek C, Paradiso PR, Siber GR. The contribution of specific pneumococcal serogroups to different disease manifestations: implications for conjugate vaccine formulation and use, part II. Clin Infect Dis. 2000 Jan;30(1):122–40. - PubMed
-
- Zangwill KM, Greenberg DP, Chiu CY, Mendelman P, Wong VK, Chang SJ, et al. Safety and immunogenicity of a heptavalent pneumococcal conjugate vaccine in infants. Vaccine. 2003 May 16;21(17-18):1894–900. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

