Abscisic acid synergizes with rosiglitazone to improve glucose tolerance and down-modulate macrophage accumulation in adipose tissue: possible action of the cAMP/PKA/PPAR γ axis
- PMID: 20207056
- PMCID: PMC2888662
- DOI: 10.1016/j.clnu.2010.02.003
Abscisic acid synergizes with rosiglitazone to improve glucose tolerance and down-modulate macrophage accumulation in adipose tissue: possible action of the cAMP/PKA/PPAR γ axis
Abstract
Background & aims: Abscisic acid (ABA) is effective in preventing insulin resistance and obesity-related inflammation through a PPAR γ-dependent mechanism. The objective of this study was to assess the efficacy ABA in improving glucose homeostasis and suppress inflammation when administered in combination with rosiglitazone (Ros) and to determine whether PPAR γ activation by ABA is initiated via cAMP/protein kinase A (PKA) signaling.
Methods: Obese db/db mice were fed high-fat diets containing 0, 10, or 70 mg/kg Ros with and without racemic ABA (100 mg/kg) for 60 days. Glucose tolerance and fasting insulin levels were assessed at 6 and 8 weeks, respectively, and adipose tissue macrophage (ATM) infiltration was examined by flow cytometry. Gene expression was examined on white adipose tissue (WAT) and stromal vascular cells (SVCs) cultured with ABA, Ros, or an ABA/Ros combination.
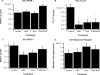
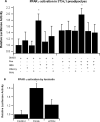
Results: Both Ros and ABA improved glucose tolerance, and ABA decreased plasma insulin levels while having no effect on Ros-induced weight gain. ABA in combination with low-dose Ros (10 mg/kg; Roslo) synergistically inhibited ATM infiltration. Treatment of SVCs with Ros, ABA or ABA/Ros suppressed expression of the M1 marker CCL17. ABA and Ros synergistically increased PPAR γ activity and pretreatment with a cAMP-inhibitor or a PKA-inhibitor abrogated ABA-induced PPAR γ activation.
Conclusions: ABA and Ros act synergistically to modulate PPAR γ activity and macrophage accumulation in WAT and ABA enhances PPAR γ activity through a membrane-initiated mechanism dependent on cAMP/PKA signaling.
Copyright © 2010 Elsevier Ltd and European Society for Clinical Nutrition and Metabolism. All rights reserved.
Figures


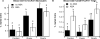

Similar articles
-
Dietary abscisic acid ameliorates glucose tolerance and obesity-related inflammation in db/db mice fed high-fat diets.Clin Nutr. 2007 Feb;26(1):107-16. doi: 10.1016/j.clnu.2006.07.008. Epub 2006 Sep 26. Clin Nutr. 2007. PMID: 17000034
-
Loss of PPAR gamma in immune cells impairs the ability of abscisic acid to improve insulin sensitivity by suppressing monocyte chemoattractant protein-1 expression and macrophage infiltration into white adipose tissue.J Nutr Biochem. 2008 Apr;19(4):216-28. doi: 10.1016/j.jnutbio.2007.02.010. Epub 2007 Jul 6. J Nutr Biochem. 2008. PMID: 17618105
-
MiR-130b promotes obesity associated adipose tissue inflammation and insulin resistance in diabetes mice through alleviating M2 macrophage polarization via repression of PPAR-γ.Immunol Lett. 2016 Dec;180:1-8. doi: 10.1016/j.imlet.2016.10.004. Epub 2016 Oct 13. Immunol Lett. 2016. PMID: 27746169
-
Review: Peroxisome proliferator-activated receptor gamma and adipose tissue--understanding obesity-related changes in regulation of lipid and glucose metabolism.J Clin Endocrinol Metab. 2007 Feb;92(2):386-95. doi: 10.1210/jc.2006-1268. Epub 2006 Dec 5. J Clin Endocrinol Metab. 2007. PMID: 17148564 Review.
-
Abscisic Acid: A Conserved Hormone in Plants and Humans and a Promising Aid to Combat Prediabetes and the Metabolic Syndrome.Nutrients. 2020 Jun 9;12(6):1724. doi: 10.3390/nu12061724. Nutrients. 2020. PMID: 32526875 Free PMC article. Review.
Cited by
-
Dietary abscisic acid ameliorates influenza-virus-associated disease and pulmonary immunopathology through a PPARγ-dependent mechanism.J Nutr Biochem. 2013 Jun;24(6):1019-27. doi: 10.1016/j.jnutbio.2012.07.010. Epub 2012 Sep 17. J Nutr Biochem. 2013. PMID: 22995385 Free PMC article.
-
Loss of p53 Sensitizes Cells to Palmitic Acid-Induced Apoptosis by Reactive Oxygen Species Accumulation.Int J Mol Sci. 2019 Dec 12;20(24):6268. doi: 10.3390/ijms20246268. Int J Mol Sci. 2019. PMID: 31842349 Free PMC article.
-
Regulation of CD19 CAR-T cell activation based on an engineered downstream transcription factor.Mol Ther Oncolytics. 2023 Apr 26;29:77-90. doi: 10.1016/j.omto.2023.04.005. eCollection 2023 Jun 15. Mol Ther Oncolytics. 2023. PMID: 37223115 Free PMC article.
-
Age-related impairment in insulin release: the essential role of β(2)-adrenergic receptor.Diabetes. 2012 Mar;61(3):692-701. doi: 10.2337/db11-1027. Epub 2012 Feb 7. Diabetes. 2012. PMID: 22315324 Free PMC article.
-
Antidepressant effects of abscisic acid mediated by the downregulation of corticotrophin-releasing hormone gene expression in rats.Int J Neuropsychopharmacol. 2014 Oct 31;18(4):pyu006. doi: 10.1093/ijnp/pyu006. Int J Neuropsychopharmacol. 2014. PMID: 25552429 Free PMC article.
References
-
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the united states, 1999-2004. JAMA. 2006;295:1549–55. - PubMed
-
- Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: Payer- and service-specific estimates. Health Aff (Millwood) 2009 - PubMed
-
- Bassaganya-Riera J, Skoneczka J, Kingston DGJ, et al. Mechanisms of action and medicinal applications of abscisic acid. Current Medicinal Chemistry. 2009 In press. - PubMed
-
- Guri AJ, Hontecillas R, Si H, Liu D, Bassaganya-Riera J. Dietary abscisic acid ameliorates glucose tolerance and obesity-related inflammation in db/db mice fed high-fat diets. Clin Nutr. 2007;26:107–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

