Eccentric muscle contraction and stretching evoke mechanical hyperalgesia and modulate CGRP and P2X(3) expression in a functionally relevant manner
- PMID: 20207080
- PMCID: PMC2860684
- DOI: 10.1016/j.pain.2010.02.022
Eccentric muscle contraction and stretching evoke mechanical hyperalgesia and modulate CGRP and P2X(3) expression in a functionally relevant manner
Abstract
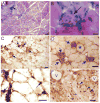
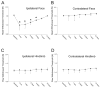
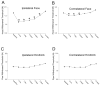
Non-invasive, movement-based models were used to investigate muscle pain. In rats, the masseter muscle was rapidly stretched or electrically stimulated during forced lengthening to produce eccentric muscle contractions (EC). Both EC and stretching disrupted scattered myofibers and produced intramuscular plasma extravasation. Pro-inflammatory cytokines (IL-1beta, TNF-alpha, IL-6) and vascular endothelial growth factor (VEGF) were elevated in the masseter 24h following EC. At 48h, neutrophils increased and ED1 macrophages infiltrated myofibers while ED2 macrophages were abundant at 4d. Mechanical hyperalgesia was evident in the ipsilateral head 4h-4d after a single bout of EC and for 7d following multiple bouts (1 bout/d for 4d). Calcitonin gene-related peptide (CGRP) mRNA increased in the trigeminal ganglion 24h following EC while immunoreactive CGRP decreased. By 2d, CGRP-muscle afferent numbers equaled naive numbers implying that CGRP is released following EC and replenished within 2d. EC elevated P2X(3) mRNA and increased P2X(3) muscle afferent neuron number for 12d while electrical stimulation without muscle contraction altered neither CGRP nor P2X(3) mRNA levels. Muscle stretching produced hyperalgesia for 2d whereas contraction alone produced no hyperalgesia. Stretching increased CGRP mRNA at 24h but not CGRP-muscle afferent number at 2-12d. In contrast, stretching significantly increased the number of P2X(3) muscle afferent neurons for 12d. The sustained, elevated P2X(3) expression evoked by EC and stretching may enhance nociceptor responsiveness to ATP released during subsequent myofiber damage. Movement-based actions such as EC and muscle stretching produce unique tissue responses and modulate neuropeptide and nociceptive receptor expression in a manner particularly relevant to repeated muscle damage.
Copyright 2010 International Association for the Study of Pain. Published by Elsevier B.V. All rights reserved.
Figures




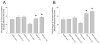
Similar articles
-
Mechanisms mediating the enhanced gene transcription of P2X3 receptor by calcitonin gene-related peptide in trigeminal sensory neurons.J Biol Chem. 2008 Jul 4;283(27):18743-52. doi: 10.1074/jbc.M800296200. Epub 2008 May 6. J Biol Chem. 2008. PMID: 18460469
-
Muscle inflammation induces a rapid increase in calcitonin gene-related peptide (CGRP) mRNA that temporally relates to CGRP immunoreactivity and nociceptive behavior.Neuroscience. 2006 Dec;143(3):875-84. doi: 10.1016/j.neuroscience.2006.08.015. Epub 2006 Oct 4. Neuroscience. 2006. PMID: 17027165
-
Trigeminal P2X3 receptor expression differs from dorsal root ganglion and is modulated by deep tissue inflammation.Pain. 2005 Oct;117(3):280-291. doi: 10.1016/j.pain.2005.06.029. Pain. 2005. PMID: 16153775
-
Molecular mechanisms of sensitization of pain-transducing P2X3 receptors by the migraine mediators CGRP and NGF.Mol Neurobiol. 2008 Feb;37(1):83-90. doi: 10.1007/s12035-008-8020-5. Epub 2008 May 6. Mol Neurobiol. 2008. PMID: 18459072 Review.
-
Emerging peripheral receptor targets for deep-tissue craniofacial pain therapies.J Dent Res. 2009 Mar;88(3):201-11. doi: 10.1177/0022034508330176. J Dent Res. 2009. PMID: 19329451 Free PMC article. Review.
Cited by
-
Dual Modulation of Nociception and Cardiovascular Reflexes during Peripheral Ischemia through P2Y1 Receptor-Dependent Sensitization of Muscle Afferents.J Neurosci. 2016 Jan 6;36(1):19-30. doi: 10.1523/JNEUROSCI.2856-15.2016. J Neurosci. 2016. PMID: 26740646 Free PMC article.
-
Novice lifters exhibit a more kyphotic lifting posture than experienced lifters in straight-leg lifting.J Biomech. 2015 Jul 16;48(10):1693-9. doi: 10.1016/j.jbiomech.2015.05.022. Epub 2015 Jun 4. J Biomech. 2015. PMID: 26077846 Free PMC article.
-
Beyond CGRP: The calcitonin peptide family as targets for migraine and pain.Br J Pharmacol. 2022 Feb;179(3):381-399. doi: 10.1111/bph.15605. Epub 2021 Jul 27. Br J Pharmacol. 2022. PMID: 34187083 Free PMC article. Review.
-
Periorbital botulinum toxin A improves photophobia and sensations of dryness in patients without migraine: Case series of four patients.Am J Ophthalmol Case Rep. 2020 Jul 4;19:100809. doi: 10.1016/j.ajoc.2020.100809. eCollection 2020 Sep. Am J Ophthalmol Case Rep. 2020. PMID: 32671286 Free PMC article.
-
P2X3 receptors contribute to muscle pain induced by static contraction by a mechanism dependent on neutrophil migration.Purinergic Signal. 2019 Jun;15(2):167-175. doi: 10.1007/s11302-019-09659-0. Epub 2019 May 21. Purinergic Signal. 2019. PMID: 31115830 Free PMC article.
References
-
- Ambalavanar R, Dessem D. The involvement of calcitonin gene related peptide in deep tissue nociception. In: Farley Elizabeth P., editor. Progress in Neuropeptide Research. New York: NOVA; 2007.
-
- Ambalavanar R, Moritani M, Dessem D. Trigeminal P2X3 receptor expression differs from dorsal root ganglion and is modulated by deep tissue inflammation. Pain. 2005;117:280–291. - PubMed
-
- Ambalavanar R, Moritani M, Haines A, Hilton T, Dessem D. Chemical phenotypes of muscle and cutaneous afferent neurons in the rat trigeminal ganglion. J Comp Neurol. 2003;460:167–179. - PubMed
-
- Ambalavanar R, Moritani M, Moutanni A, Gangula P, Yallampalli C, Dessem D. Deep tissue inflammation upregulates neuropeptides and evokes nociceptive behaviors which are modulated by a neuropeptide antagonist. Pain. 2006;120:53–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

