Oxime esters as selective, covalent inhibitors of the serine hydrolase retinoblastoma-binding protein 9 (RBBP9)
- PMID: 20207142
- PMCID: PMC2843764
- DOI: 10.1016/j.bmcl.2010.02.011
Oxime esters as selective, covalent inhibitors of the serine hydrolase retinoblastoma-binding protein 9 (RBBP9)
Abstract
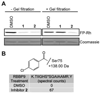
We recently described a fluorescence polarization platform for competitive activity-based protein profiling (fluopol-ABPP) that enables high-throughput inhibitor screening for enzymes with poorly characterized biochemical activity. Here, we report the discovery of a class of oxime ester inhibitors for the unannotated serine hydrolase RBBP9 from a full-deck (200,000+ compound) fluopol-ABPP screen conducted in collaboration with the Molecular Libraries Screening Center Network (MLSCN). We show that these compounds covalently inhibit RBBP9 by modifying enzyme's active site serine nucleophile and, based on competitive ABPP in cell and tissue proteomes, are selective for RBBP9 relative to other mammalian serine hydrolases.
2010 Elsevier Ltd. All rights reserved.
Figures
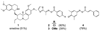


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information

