Endothelin-1 production by the canine macrophage cell line DH82: enhanced production in response to microbial challenge
- PMID: 20207425
- PMCID: PMC2892245
- DOI: 10.1016/j.vetimm.2010.02.006
Endothelin-1 production by the canine macrophage cell line DH82: enhanced production in response to microbial challenge
Abstract
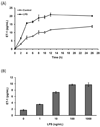
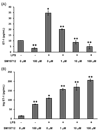
Endothelin-1 (ET-1) is a potent vasoconstrictive peptide which plays an important role in regulating mammalian cardiovascular development and homeostasis. Originally identified as a factor released by vascular endothelial cells, ET-1 is now recognized as a product of numerous cells and tissues with demonstrated involvement in an array of physiological and pathological processes. An area of great interest is the production of ET-1 by mononuclear cells (monocytes and macrophages) and its role in inflammation. We report that the canine macrophage cell line, DH82, constitutively secretes both ET-1 and its biologically inactive precursor big ET-1. The production of both peptides was increased following stimulation with lipopolysaccharide (endotoxin) from gram-negative bacteria. ET-1 production was also increased in response to stimulation with intact and viable gram-positive and gram-negative bacteria. In addition to producing ET-1, DH82 cells express transcripts encoding two receptors for the ET-1 peptide (ET(A) and ET(B) receptors) and an enzyme involved in the conversion of big ET-1 to ET-1. The constitutive secretion of ET-1 and the expression of ET(A) and ET(B) receptors may be related to the malignant origin of this cell line. Our results are the first report of ET-1 production by a canine cell line and provide the basis for further investigation into the role of ET-1 during infection and inflammation.
Copyright 2010 Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Differential regulation of the lung endothelin system by urban particulate matter and ozone.Toxicol Sci. 2005 Nov;88(1):103-13. doi: 10.1093/toxsci/kfi272. Epub 2005 Aug 4. Toxicol Sci. 2005. PMID: 16081523
-
Expression of endothelin-1 system in a pig model of endotoxic shock.Regul Pept. 2005 Nov;131(1-3):89-96. doi: 10.1016/j.regpep.2005.07.001. Regul Pept. 2005. PMID: 16043243
-
Increase in tissue endothelin-1 and ETA receptor levels in human aortic valve stenosis.Eur Heart J. 2009 Jan;30(2):242-9. doi: 10.1093/eurheartj/ehn482. Epub 2008 Nov 13. Eur Heart J. 2009. PMID: 19008257
-
The endothelin axis: a novel target for pharmacotherapy of female malignancies.Curr Vasc Pharmacol. 2007 Jul;5(3):239-48. doi: 10.2174/157016107781024082. Curr Vasc Pharmacol. 2007. PMID: 17627567 Review.
-
Endothelin@25 - new agonists, antagonists, inhibitors and emerging research frontiers: IUPHAR Review 12.Br J Pharmacol. 2014 Dec;171(24):5555-72. doi: 10.1111/bph.12874. Epub 2014 Nov 24. Br J Pharmacol. 2014. PMID: 25131455 Free PMC article. Review.
Cited by
-
Aspirin Caused Intestinal Damage through FXR and ET-1 Signaling Pathways.Int J Mol Sci. 2024 Mar 18;25(6):3424. doi: 10.3390/ijms25063424. Int J Mol Sci. 2024. PMID: 38542397 Free PMC article.
-
Decreased Ambient Oxygen Tension Alters the Expression of Endothelin-1, iNOS and cGMP in Rat Alveolar Macrophages.Int J Med Sci. 2019 Feb 28;16(3):443-449. doi: 10.7150/ijms.28353. eCollection 2019. Int J Med Sci. 2019. PMID: 30911278 Free PMC article.
-
A novel role of endothelin-1 in linking Toll-like receptor 7-mediated inflammation to fibrosis in congenital heart block.J Biol Chem. 2011 Sep 2;286(35):30444-30454. doi: 10.1074/jbc.M111.263657. Epub 2011 Jul 5. J Biol Chem. 2011. PMID: 21730058 Free PMC article.
-
Cellular Microbiology of Mycoplasma canis.Infect Immun. 2016 May 24;84(6):1785-1795. doi: 10.1128/IAI.01440-15. Print 2016 Jun. Infect Immun. 2016. PMID: 27045036 Free PMC article.
References
-
- Battistini B, Forget MA, Laight D. Potential Roles for Endothelins in Systemic Inflammatory Response Syndrome with a Particular Relationship to Cytokines. Shock. 1996;5:167–183. - PubMed
-
- Best PJ, Lerman A. Endothelin in Cardiovascular Disease: From Atherosclerosis to Heart Failure. J. Cardiovasc. Pharmacol. 2000;35:S61–S63. - PubMed
-
- Clouthier DE, Hosoda K, Richardson JA, Williams SC, Yanagisawa H, Kuwaki T, Kumada M, Hammer RE, Yanagisawa M. Cranial and Cardiac Neural Crest Defects in Endothelin-A Receptor-Deficient Mice. Development. 1998;125:813–824. - PubMed
-
- Ebihara I, Nakamura T, Shimada N, Shoji H, Koide H. Effect of Hemoperfusion with Polymyxin B-Immobilized Fiber on Plasma Endothelin-1 and Endothelin-1 mRNA in Monocytes from Patients with Sepsis. Am. J. Kidney Dis. 1998;32:953–961. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

