Rapid testing for malaria in settings where microscopy is available and peripheral clinics where only presumptive treatment is available: a randomised controlled trial in Ghana
- PMID: 20207689
- PMCID: PMC2833239
- DOI: 10.1136/bmj.c930
Rapid testing for malaria in settings where microscopy is available and peripheral clinics where only presumptive treatment is available: a randomised controlled trial in Ghana
Abstract
Objective: To test in West Africa the impact of rapid diagnostic tests on the prescription of antimalarials and antibiotics both where microscopy is used for the diagnosis of malaria and in clinical (peripheral) settings that rely on clinical diagnosis.
Design: Randomised, controlled, open label clinical trial.
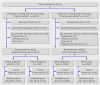
Setting: Four clinics in the rural Dangme West district of southern Ghana, one in which microscopy is used for diagnosis of malaria ("microscopy setting") and three where microscopy is not available and diagnosis of malaria is made on the basis of clinical symptoms ("clinical setting").
Participants: Patients with suspected malaria. Interventions Patients were randomly assigned to either a rapid diagnostic test or the current diagnostic method at the clinic (microscopy or clinical diagnosis). A blood sample for a research microscopy slide was taken for all patients.
Main outcome measures: The primary outcome was the prescription of antimalarials to patients of any age whose double read research slide was negative for malaria. The major secondary outcomes were the correct prescription of antimalarials, the impact of test results on antibiotic prescription, and the correct prescription of antimalarials in children under 5 years.
Results: Of the 9236 patients screened, 3452 were randomised in the clinical setting and 3811 in the microscopy setting. Follow-up to 28 days was 97.6% (7088/7263). In the microscopy setting, 722 (51.6%) of the 1400 patients with negative research slides in the rapid diagnostic test arm were treated for malaria compared with 764 (55.0%) of the 1389 patients in the microscopy arm (adjusted odds ratio 0.87, 95% CI 0.71 to 1.1; P=0.16). In the clinical setting, 578 (53.9%) of the 1072 patients in the rapid diagnostic test arm with negative research slides were treated for malaria compared with 982 (90.1%) of the 1090 patients with negative slides in the clinical diagnosis arm (odds ratio 0.12, 95% CI 0.04 to 0.38; P=0.001). The use of rapid diagnostic tests led to better targeting of antimalarials and antibiotics in the clinical but not the microscopy setting, in both children and adults. There were no deaths in children under 5 years at 28 days follow-up in either arm.
Conclusion: Where microscopy already exists, introducing rapid diagnostic tests had limited impact on prescriber behaviour. In settings where microscopy was not available, however, using rapid diagnostic tests led to a significant reduction in the overprescription of antimalarials, without any evidence of clinical harm, and to better targeting of antibiotics. Trial registration ClinicalTrials.gov NCT00493922.
Conflict of interest statement
Competing interests: All authors have completed the Unified Competing Interest form at
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical