Increased sensorimotor network activity in DYT1 dystonia: a functional imaging study
- PMID: 20207699
- PMCID: PMC2842516
- DOI: 10.1093/brain/awq017
Increased sensorimotor network activity in DYT1 dystonia: a functional imaging study
Abstract
Neurophysiological studies have provided evidence of primary motor cortex hyperexcitability in primary dystonia, but several functional imaging studies suggest otherwise. To address this issue, we measured sensorimotor activation at both the regional and network levels in carriers of the DYT1 dystonia mutation and in control subjects. We used (15)Oxygen-labelled water and positron emission tomography to scan nine manifesting DYT1 carriers, 10 non-manifesting DYT1 carriers and 12 age-matched controls while they performed a kinematically controlled motor task; they were also scanned in a non-motor audio-visual control condition. Within- and between-group contrasts were analysed with statistical parametric mapping. For network analysis, we first identified a normal motor-related activation pattern in a set of 39 motor and audio-visual scans acquired in an independent cohort of 18 healthy volunteer subjects. The expression of this pattern was prospectively quantified in the motor and control scans acquired in each of the gene carriers and controls. Network values for the three groups were compared with ANOVA and post hoc contrasts. Voxel-wise comparison of DYT1 carriers and controls revealed abnormally increased motor activation responses in the former group (P < 0.05, corrected; statistical parametric mapping), localized to the sensorimotor cortex, dorsal premotor cortex, supplementary motor area and the inferior parietal cortex. Network analysis of the normative derivation cohort revealed a significant normal motor-related activation pattern topography (P < 0.0001) characterized by covarying neural activity in the sensorimotor cortex, dorsal premotor cortex, supplementary motor area and cerebellum. In the study cohort, normal motor-related activation pattern expression measured during movement was abnormally elevated in the manifesting gene carriers (P < 0.001) but not in their non-manifesting counterparts. In contrast, in the non-motor control condition, abnormal increases in network activity were present in both groups of gene carriers (P < 0.001). In this condition, normal motor-related activation pattern expression in non-manifesting carriers was greater than in controls, but lower than in affected carriers. In the latter group, measures of normal motor-related activation pattern expression in the audio-visual condition correlated with independent dystonia clinical ratings (r = 0.70, P = 0.04). These findings confirm that overexcitability of the sensorimotor system is a robust feature of dystonia. The presence of elevated normal motor-related activation pattern expression in the non-motor condition suggests that abnormal integration of audio-visual input with sensorimotor network activity is an important trait feature of this disorder. Lastly, quantification of normal motor-related activation pattern expression in individual cases may have utility as an objective descriptor of therapeutic response in trials of new treatments for dystonia and related disorders.
Figures
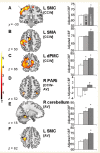
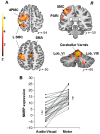
 PET scans (39 CCW- and 39 AV) acquired in 18 healthy volunteer subjects (see text). This spatial covariance pattern was characterized by activation of the left sensorimotor cortex (SMC), premotor cortex (dPMC) and inferior parietal cortex (Table 3). Additional regional contributions to network activity were found bilaterally in the cerebellar vermis and hemispheres. [The colour scale represents positive voxel weights thresholded at Z = 3.09, corresponding to regions that contributed significantly (P < 0.001) to network activity and which were also reliable (P < 0.001) on bootstrap estimation.] (B) Normal motor-related activation pattern expression in the subjects comprising the original derivation sample. For all subjects and runs, the expression of this pattern increased during the performance of the motor task (P < 0.0001; see text).
PET scans (39 CCW- and 39 AV) acquired in 18 healthy volunteer subjects (see text). This spatial covariance pattern was characterized by activation of the left sensorimotor cortex (SMC), premotor cortex (dPMC) and inferior parietal cortex (Table 3). Additional regional contributions to network activity were found bilaterally in the cerebellar vermis and hemispheres. [The colour scale represents positive voxel weights thresholded at Z = 3.09, corresponding to regions that contributed significantly (P < 0.001) to network activity and which were also reliable (P < 0.001) on bootstrap estimation.] (B) Normal motor-related activation pattern expression in the subjects comprising the original derivation sample. For all subjects and runs, the expression of this pattern increased during the performance of the motor task (P < 0.0001; see text).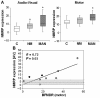
References
-
- Alterman RL, Miravite J, Weisz D, Shils JL, Bressman SB, Tagliati M. Sixty hertz pallidal deep brain stimulation for primary torsion dystonia. Neurology. 2007;69:681–8. - PubMed
-
- Berardelli A, Rothwell JC, Hallett M, Thompson PD, Manfredi M, Marsden CD. The pathophysiology of primary dystonia. Brain. 1998;121:1195–212. - PubMed
-
- Blood AJ, Flaherty AW, Choi JK, Hochberg FH, Greve DN, Bonmassar G, et al. Basal ganglia activity remains elevated after movement in focal hand dystonia. Ann Neurol. 2004;55:744–8. - PubMed
-
- Buneo CA, Jarvis MR, Batista AP, Andersen RA. Direct visuomotor transformations for reaching. Nature. 2002;416:632–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

