Role of the netrin-like domain of procollagen C-proteinase enhancer-1 in the control of metalloproteinase activity
- PMID: 20207734
- PMCID: PMC2871463
- DOI: 10.1074/jbc.M109.086447
Role of the netrin-like domain of procollagen C-proteinase enhancer-1 in the control of metalloproteinase activity
Abstract
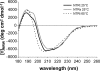
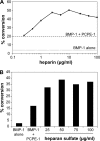
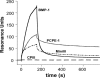
The netrin-like (NTR) domain is a feature of several extracellular proteins, most notably the N-terminal domain of tissue inhibitors of metalloproteinases (TIMPs), where it functions as a strong inhibitor of matrix metalloproteinases and some other members of the metzincin superfamily. The presence of a C-terminal NTR domain in procollagen C-proteinase enhancers (PCPEs), proteins that stimulate the activity of astacin-like tolloid proteinases, raises the possibility that this might also have inhibitory activity. Here we show that both long and short forms of the PCPE-1 NTR domain, the latter beginning at the N-terminal cysteine known to be critical for TIMP activity, show no inhibition, at micromolar concentrations, of several members of the metzincin superfamily, including matrix metalloproteinase-2, bone morphogenetic protein-1 (a tolloid proteinase), and different ADAMTS (a disintegrin and a metalloproteinase with thrombospondin motifs) proteinases from the adamalysin family. In contrast, we report that the NTR domain within PCPE-1 leads to superstimulation of bone morphogenetic protein-1 activity in the presence of heparin and heparan sulfate. These observations point to a new mechanism whereby binding to cell surface-associated or extracellular heparin-like sulfated glycosaminoglycans might provide a means to accelerate procollagen processing in specific cellular and extracellular microenvironments.
Figures

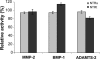



Similar articles
-
Identification of the minimal domain structure of bone morphogenetic protein-1 (BMP-1) for chordinase activity: chordinase activity is not enhanced by procollagen C-proteinase enhancer-1 (PCPE-1).J Biol Chem. 2005 Jun 17;280(24):22616-23. doi: 10.1074/jbc.M413468200. Epub 2005 Apr 7. J Biol Chem. 2005. PMID: 15817489
-
The NTR domain of procollagen C-proteinase enhancer-1 (PCPE-1) mediates PCPE-1 binding to syndecans-1, -2 and -4 as well as fibronectin.Int J Biochem Cell Biol. 2014 Dec;57:45-53. doi: 10.1016/j.biocel.2014.09.023. Epub 2014 Oct 5. Int J Biochem Cell Biol. 2014. PMID: 25286301
-
Binding of procollagen C-proteinase enhancer-1 (PCPE-1) to heparin/heparan sulfate: properties and role in PCPE-1 interaction with cells.J Biol Chem. 2010 Oct 29;285(44):33867-74. doi: 10.1074/jbc.M110.141366. Epub 2010 Aug 21. J Biol Chem. 2010. PMID: 20729553 Free PMC article.
-
PCPE-2 (procollagen C-proteinase enhancer-2): The non-identical twin of PCPE-1.Matrix Biol. 2024 Dec;134:59-78. doi: 10.1016/j.matbio.2024.09.001. Epub 2024 Sep 7. Matrix Biol. 2024. PMID: 39251075 Review.
-
The Role of the Metzincin Superfamily in Prostate Cancer Progression: A Systematic-Like Review.Int J Mol Sci. 2021 Mar 30;22(7):3608. doi: 10.3390/ijms22073608. Int J Mol Sci. 2021. PMID: 33808504 Free PMC article.
Cited by
-
Fibronectin matrix as a scaffold for procollagen proteinase binding and collagen processing.Mol Biol Cell. 2019 Aug 1;30(17):2218-2226. doi: 10.1091/mbc.E19-03-0140. Epub 2019 Jun 26. Mol Biol Cell. 2019. PMID: 31242089 Free PMC article.
-
Procollagen C-proteinase enhancer 1 promotes physiologic retinal angiogenesis via regulating the process of collagen.Int J Ophthalmol. 2022 Jun 18;15(6):868-875. doi: 10.18240/ijo.2022.06.03. eCollection 2022. Int J Ophthalmol. 2022. PMID: 35814888 Free PMC article.
-
Taking the Occam's Razor Approach to Hedgehog Lipidation and Its Role in Development.J Dev Biol. 2018 Jan 30;6(1):3. doi: 10.3390/jdb6010003. J Dev Biol. 2018. PMID: 29615552 Free PMC article. Review.
-
Synthetic enzyme-substrate tethering obviates the Tolloid-ECM interaction during Drosophila BMP gradient formation.Elife. 2015 Feb 2;4:e05508. doi: 10.7554/eLife.05508. Elife. 2015. PMID: 25642644 Free PMC article.
-
Biomineralization of bone: a fresh view of the roles of non-collagenous proteins.Front Biosci (Landmark Ed). 2011 Jun 1;16(7):2598-621. doi: 10.2741/3875. Front Biosci (Landmark Ed). 2011. PMID: 21622198 Free PMC article. Review.
References
-
- Nagase H., Visse R., Murphy G. (2006) Cardiovasc. Res. 69, 562–573 - PubMed
-
- Stetefeld J., Jenny M., Schulthess T., Landwehr R., Schumacher B., Frank S., Rüegg M. A., Engel J., Kammerer R. A. (2001) Nat. Struct. Biol. 8, 705–709 - PubMed
-
- Bezakova G., Ruegg M. A. (2003) Nat. Rev. Mol. Cell Biol. 4, 295–308 - PubMed
-
- Cirulli V., Yebra M. (2007) Nat. Rev. Mol. Cell Biol. 8, 296–306 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

