Isoproterenol and cAMP block ERK phosphorylation and enhance [Ca2+]i increases and oxygen consumption by muscarinic receptor stimulation in rat parotid and submandibular acinar cells
- PMID: 20207737
- PMCID: PMC2859492
- DOI: 10.1074/jbc.M110.112094
Isoproterenol and cAMP block ERK phosphorylation and enhance [Ca2+]i increases and oxygen consumption by muscarinic receptor stimulation in rat parotid and submandibular acinar cells
Abstract
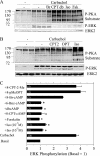
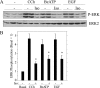
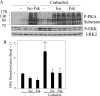
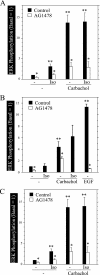
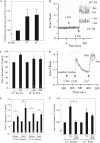
Salivary glands are innervated by sympathetic and parasympathetic neurons, which release neurotransmitters that promote fluid secretion and exocytosis when they bind to muscarinic and beta-adrenergic receptors, respectively. Signaling pathways downstream of these receptors are mainly distinct, but there is cross-talk that affects receptor-dependent events. Here we report that the beta-adrenergic ligand isoproterenol blocks increases in extracellular signal-related kinase (ERK) phosphorylation, a protein kinase C-dependent event promoted by the muscarinic receptor ligand carbachol in freshly dispersed rat parotid acinar cells. The inhibitory action of isoproterenol was reproduced by cAMP stimuli (forskolin) and mimetics (dibutyryl-cAMP, 8-(4-chlorophenylthio)-cAMP), including one highly selective for protein kinase A (N(6)-benzoyl-cAMP). In contrast, Epac (exchange proteins directly activated by cAMP)-selective activators did not mimic the blockade of ERK by isoproterenol, suggesting that inhibition involved protein kinase A. Isoproterenol also blocked ERK downstream of phorbol 12-myristate 13-acetate and the P2X(7) and epidermal growth factor receptors. Isoproterenol and forskolin blocked MEK phosphorylation, reduced RAF phosphorylation on a stimulatory site (Ser-338), and increased RAF phosphorylation on an inhibitory site (Ser-259). Inhibitory effects on ERK were also observed in freshly dispersed rat submandibular acinar cells but not in three immortalized/cancer salivary cell lines (Par-C10, HSY, HSG), indicating significant differences between native cells and cell lines. Notably, in native parotid cells isoproterenol enhanced the carbachol-promoted increases in [Ca(2+)](i) and oxygen consumption, events that initiate and accompany, respectively, the stimulation of fluid secretion by muscarinic ligands. Thus, isoproterenol produces opposite effects on prominent events downstream of the muscarinic receptor second messengers diacylglycerol (decrease in ERK phosphorylation) and inositol trisphosphate (increase in [Ca(2+)](i) and fluid secretion).
Figures

References
-
- Proctor G. B., Carpenter G. H. (2007) Auton. Neurosci. 133, 3–18 - PubMed
-
- Dai Y. S., Ambudkar I. S., Horn V. J., Yeh C. K., Kousvelari E. E., Wall S. J., Li M., Yasuda R. P., Wolfe B. B., Baum B. J. (1991) Am. J. Physiol. 261, C1063–C1073 - PubMed
-
- Melvin J. E., Yule D., Shuttleworth T., Begenisich T. (2005) Annu. Rev. Physiol. 67, 445–469 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

