Mutations of the opsin gene (Y102H and I307N) lead to light-induced degeneration of photoreceptors and constitutive activation of phototransduction in mice
- PMID: 20207741
- PMCID: PMC2863193
- DOI: 10.1074/jbc.M110.112409
Mutations of the opsin gene (Y102H and I307N) lead to light-induced degeneration of photoreceptors and constitutive activation of phototransduction in mice
Abstract
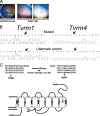
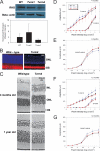
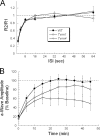
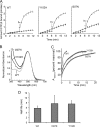
Mutations in the Rhodopsin (Rho) gene can lead to autosomal dominant retinitis pigmentosa (RP) in humans. Transgenic mouse models with mutations in Rho have been developed to study the disease. However, it is difficult to know the source of the photoreceptor (PR) degeneration in these transgenic models because overexpression of wild type (WT) Rho alone can lead to PR degeneration. Here, we report two chemically mutagenized mouse models carrying point mutations in Rho (Tvrm1 with an Y102H mutation and Tvrm4 with an I307N mutation). Both mutants express normal levels of rhodopsin that localize to the PR outer segments and do not exhibit PR degeneration when raised in ambient mouse room lighting; however, severe PR degeneration is observed after short exposures to bright light. Both mutations also cause a delay in recovery following bleaching. This defect might be due to a slower rate of chromophore binding by the mutant opsins compared with the WT form, and an increased rate of transducin activation by the unbound mutant opsins, which leads to a constitutive activation of the phototransduction cascade as revealed by in vitro biochemical assays. The mutant-free opsins produced by the respective mutant Rho genes appear to be more toxic to PRs, as Tvrm1 and Tvrm4 mutants lacking the 11-cis chromophore degenerate faster than mice expressing WT opsin that also lack the chromophore. Because of their phenotypic similarity to humans with B1 Rho mutations, these mutants will be important tools in examining mechanisms underlying Rho-induced RP and for testing therapeutic strategies.
Figures



References
-
- Rim J., Oprian D. D. (1995) Biochemistry 34, 11938–11945 - PubMed
-
- Humphries M. M., Rancourt D., Farrar G. J., Kenna P., Hazel M., Bush R. A., Sieving P. A., Sheils D. M., McNally N., Creighton P., Erven A., Boros A., Gulya K., Capecchi M. R., Humphries P. (1997) Nat. Genet. 15, 216–219 - PubMed
-
- Toda K., Bush R. A., Humphries P., Sieving P. A. (1999) Vis. Neurosci. 16, 391–398 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

