RyR1-mediated Ca2+ leak and Ca2+ entry determine resting intracellular Ca2+ in skeletal myotubes
- PMID: 20207743
- PMCID: PMC2859541
- DOI: 10.1074/jbc.M110.107300
RyR1-mediated Ca2+ leak and Ca2+ entry determine resting intracellular Ca2+ in skeletal myotubes
Abstract
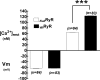
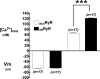
The control of resting free Ca(2+) in skeletal muscle is thought to be a balance of channels, pumps, and exchangers in both the sarcolemma and sarcoplasmic reticulum. We explored these mechanisms using pharmacologic and molecular perturbations of genetically engineered (dyspedic) muscle cells that constitutively lack expression of the skeletal muscle sarcoplasmic reticulum Ca(2+) release channels, RyR1 and RyR3. We demonstrate here that expression of RyR1 is responsible for more than half of total resting Ca(2+) concentration ([Ca(2+)](rest)) measured in wild type cells. The elevated [Ca(2+)](rest) in RyR1-expressing cells is not a result of active gating of the RyR1 channel but instead is accounted for by the RyR1 ryanodine-insensitive Ca(2+) leak conformation. In addition, we demonstrate that basal sarcolemmal Ca(2+) influx is also governed by RyR1 expression and contributes in the regulation of [Ca(2+)](rest) in skeletal myotubes.
Figures






Comment in
-
RyR1 expression and the cell boundary theorem.J Biol Chem. 2010 Aug 20;285(34):le13; author reply le14. doi: 10.1074/jbc.L110.107300. J Biol Chem. 2010. PMID: 20709761 Free PMC article. No abstract available.
References
-
- López J. R., Linares N., Pessah I. N., Allen P. D. (2005) Am. J. Physiol. Cell Physiol. 288, C606–C612 - PubMed
-
- López J. R., Medina P., Alamo L. (1987) Muscle Nerve 10, 77–79 - PubMed
-
- Yang T., Esteve E., Pessah I. N., Molinski T. F., Allen P. D., López J. R. (2007) Am. J. Physiol. Cell Physiol. 292, C1591–C1598 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

