Competition of pyruvate with physiological substrates for oxidation by the heart: implications for studies with hyperpolarized [1-13C]pyruvate
- PMID: 20207817
- PMCID: PMC2867437
- DOI: 10.1152/ajpheart.00656.2009
Competition of pyruvate with physiological substrates for oxidation by the heart: implications for studies with hyperpolarized [1-13C]pyruvate
Abstract
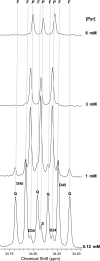
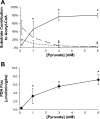
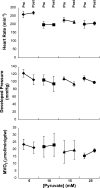
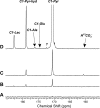
Carbon 13 nuclear magnetic resonance (NMR) isotopomer analysis was used to measure the rates of oxidation of long-chain fatty acids, ketones, and pyruvate to determine the minimum pyruvate concentration ([pyruvate]) needed to suppress oxidation of these alternative substrates. Substrate mixtures were chosen to represent either the fed or fasted state. At physiological [pyruvate], fatty acids and ketones supplied the overwhelming majority of acetyl-CoA. Under conditions mimicking the fed state, 3 mM pyruvate provided approximately 80% of acetyl-CoA, but under fasting conditions 6 mM pyruvate contributed only 33% of acetyl-CoA. Higher [pyruvate], 10-25 mM, was associated with transient reduced cardiac output, but overall hemodynamic performance was unchanged after equilibration. These observations suggested that 3-6 mM pyruvate in the coronary arteries would be an appropriate target for studies with hyperpolarized [1-(13)C]pyruvate. However, the metabolic products of 3 mM hyperpolarized [1-(13)C]pyruvate could not be detected in the isolated heart during perfusion with a physiological mixture of substrates including 3% albumin. In the presence of albumin even at high concentrations of pyruvate, 20 mM, hyperpolarized H(13)CO(3)(-) could be detected only in the absence of competing substrates. Highly purified albumin (but not albumin from plasma) substantially reduced the longitudinal relaxation time of [1-(13)C]pyruvate. In conclusion, studies of cardiac metabolism using hyperpolarized [1-(13)C]pyruvate are sensitive to the effects of competing substrates on pyruvate oxidation.
Figures


Similar articles
-
Differential modulation of glucose, lactate, and pyruvate oxidation by insulin and dichloroacetate in the rat heart.Am J Physiol Heart Circ Physiol. 2003 Jul;285(1):H163-72. doi: 10.1152/ajpheart.01117.2002. Am J Physiol Heart Circ Physiol. 2003. PMID: 12793977
-
Profiling substrate fluxes in the isolated working mouse heart using 13C-labeled substrates: focusing on the origin and fate of pyruvate and citrate carbons.Am J Physiol Heart Circ Physiol. 2004 Apr;286(4):H1461-70. doi: 10.1152/ajpheart.00942.2003. Epub 2003 Dec 11. Am J Physiol Heart Circ Physiol. 2004. PMID: 14670819
-
The cycling of acetyl-coenzyme A through acetylcarnitine buffers cardiac substrate supply: a hyperpolarized 13C magnetic resonance study.Circ Cardiovasc Imaging. 2012 Mar;5(2):201-9. doi: 10.1161/CIRCIMAGING.111.969451. Epub 2012 Jan 11. Circ Cardiovasc Imaging. 2012. PMID: 22238215 Free PMC article.
-
Fatty acid oxidation in the heart.J Cardiovasc Pharmacol. 1996;28 Suppl 1:S11-7. doi: 10.1097/00005344-199600003-00003. J Cardiovasc Pharmacol. 1996. PMID: 8891866 Review.
-
Cardiovascular applications of hyperpolarized contrast media and metabolic tracers.Exp Biol Med (Maywood). 2009 Dec;234(12):1395-416. doi: 10.3181/0904-MR-135. Exp Biol Med (Maywood). 2009. PMID: 19934362 Review.
Cited by
-
In vivo investigation of cardiac metabolism in the rat using MRS of hyperpolarized [1-13C] and [2-13C]pyruvate.NMR Biomed. 2013 Dec;26(12):1680-7. doi: 10.1002/nbm.3003. Epub 2013 Jul 31. NMR Biomed. 2013. PMID: 23904148 Free PMC article.
-
tcaSIM: A Simulation Program for Optimal Design of 13C Tracer Experiments for Analysis of Metabolic Flux by NMR and Mass Spectroscopy.Curr Metabolomics. 2018;6(3):176-187. doi: 10.2174/2213235X07666181219115856. Curr Metabolomics. 2018. PMID: 31745452 Free PMC article.
-
Resolving confounding enrichment kinetics due to overlapping resonance signals from 13C-enriched long chain fatty acid oxidation and uptake within intact hearts.Magn Reson Med. 2015 Aug;74(2):330-5. doi: 10.1002/mrm.25446. Epub 2014 Sep 8. Magn Reson Med. 2015. PMID: 25199499 Free PMC article.
-
Cardiovascular Applications of Hyperpolarized MRI.Curr Cardiovasc Imaging Rep. 2011 Apr;4(2):108-115. doi: 10.1007/s12410-011-9066-8. Epub 2011 Jan 19. Curr Cardiovasc Imaging Rep. 2011. PMID: 21475403 Free PMC article.
-
Hyperpolarized singlet lifetimes of pyruvate in human blood and in the mouse.NMR Biomed. 2013 Dec;26(12):1696-704. doi: 10.1002/nbm.3005. Epub 2013 Aug 15. NMR Biomed. 2013. PMID: 23946252 Free PMC article.
References
-
- Bae Kyongtae T, Tran Huy Q, Heiken Jay P. Uniform vascular contrast enhancement and reduced contrast medium volume achieved by using exponentially decelerated contrast material injection method. Radiology 231: 732–736, 2004 - PubMed
-
- Bassenge E, Wendt VE, Schollmeyer P, Bluemchen G, Gudbjarnason S, Bing RJ. Effect of ketone bodies on cardiac metabolism. Am J Physiol 208: 162–168, 1965 - PubMed
-
- Bing RJ, Siegel A, Ungar I, Gilbert M. Metabolism of the human heart. II. Studies on fat, ketone and amino acid metabolism. Am J Med 16: 504–515, 1954 - PubMed
-
- Bunger R, Mallet RT, Hartman DA. Pyruvate-enhanced phosphorylation potential and inotropism in normoxic and postischemic isolated working heart. Near-complete prevention of reperfusion contractile failure. Eur J Biochem 180: 221–233, 1989 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

