Lymphangiogenesis-independent resolution of experimental edema
- PMID: 20207821
- PMCID: PMC2904140
- DOI: 10.1152/ajpheart.00008.2010
Lymphangiogenesis-independent resolution of experimental edema
Abstract
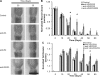
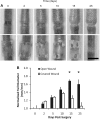
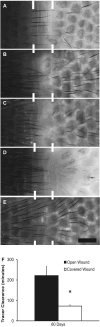
Vascular endothelial growth factor (VEGF)-C is necessary for lymphangiogenesis, and excess VEGF-C has been shown to be ameliorative for edema produced by lymphatic obstruction in experimental models. However, it has recently been shown that edema can resolve in the mouse tail even in the complete absence of capillary lymphangiogenesis when distal lymph fluid crosses the regenerating wound site interstitially. This finding has raised questions about the action of VEGF-C/VEGF receptor (VEGFR) signaling during the resolution of experimental edema. Here, the roles of VEGFR-2 and VEGFR-3 signaling in edema resolution were explored. It was found that edema resolved following neutralization of either VEGFR-2 or VEGFR-3 in the mouse tail skin, which inhibited lymphangiogenesis. Neutralization of either VEGFR-2 or VEGFR-3 reduced angiogenesis at the site of obstruction at day 10 (9.2 +/- 1.2% and 11.5 +/- 1.0% blood capillary coverage, respectively) relative to controls (14.3 +/- 1.5% blood capillary coverage). Combined VEGFR-2/-3 neutralization more strongly inhibited angiogenesis (6.9 +/- 1.5% blood capillary coverage), leading to a reduced wound repair of the lymphatic obstruction and extended edema in the tail skin. In contrast, improved tissue repair of the obstruction site increased edema resolution. Macrophages in the swollen tissue were excluded as contributing factors in the VEGFR-dependent extended edema. These results support a role for VEGFR-2/-3-combined signaling in the resolution of experimental edema that is lymphangiogenesis independent.
Figures


Similar articles
-
The resolution of lymphedema by interstitial flow in the mouse tail skin.Am J Physiol Heart Circ Physiol. 2008 Mar;294(3):H1326-34. doi: 10.1152/ajpheart.00900.2007. Epub 2008 Jan 18. Am J Physiol Heart Circ Physiol. 2008. PMID: 18203849
-
Sunitinib inhibits lymphatic endothelial cell functions and lymph node metastasis in a breast cancer model through inhibition of vascular endothelial growth factor receptor 3.Breast Cancer Res. 2011 Jun 21;13(3):R66. doi: 10.1186/bcr2903. Breast Cancer Res. 2011. PMID: 21693010 Free PMC article.
-
Differential Receptor Binding and Regulatory Mechanisms for the Lymphangiogenic Growth Factors Vascular Endothelial Growth Factor (VEGF)-C and -D.J Biol Chem. 2016 Dec 30;291(53):27265-27278. doi: 10.1074/jbc.M116.736801. Epub 2016 Nov 16. J Biol Chem. 2016. PMID: 27852824 Free PMC article.
-
Molecular control of lymphatic metastasis.Ann N Y Acad Sci. 2008;1131:225-34. doi: 10.1196/annals.1413.020. Ann N Y Acad Sci. 2008. PMID: 18519975 Review.
-
VEGFR signaling during lymphatic vascular development: From progenitor cells to functional vessels.Dev Dyn. 2015 Mar;244(3):323-31. doi: 10.1002/dvdy.24227. Epub 2014 Dec 4. Dev Dyn. 2015. PMID: 25399804 Review.
Cited by
-
A chronic and latent lymphatic insufficiency follows recovery from acute lymphedema in the rat foreleg.Am J Physiol Heart Circ Physiol. 2012 Nov 1;303(9):H1107-13. doi: 10.1152/ajpheart.00522.2012. Epub 2012 Aug 31. Am J Physiol Heart Circ Physiol. 2012. PMID: 22942182 Free PMC article.
-
A 3D biomimetic model of lymphatics reveals cell-cell junction tightening and lymphedema via a cytokine-induced ROCK2/JAM-A complex.Proc Natl Acad Sci U S A. 2023 Oct 10;120(41):e2308941120. doi: 10.1073/pnas.2308941120. Epub 2023 Oct 2. Proc Natl Acad Sci U S A. 2023. PMID: 37782785 Free PMC article.
-
Fibrosis worsens chronic lymphedema in rodent tissues.Am J Physiol Heart Circ Physiol. 2015 May 15;308(10):H1229-36. doi: 10.1152/ajpheart.00527.2013. Epub 2015 Mar 13. Am J Physiol Heart Circ Physiol. 2015. PMID: 25770241 Free PMC article.
-
A new lymphedema treatment using pyro-drive jet injection.Hum Cell. 2024 Mar;37(2):465-477. doi: 10.1007/s13577-023-01021-2. Epub 2024 Jan 14. Hum Cell. 2024. PMID: 38218753
-
VEGFR-3 blocking deteriorates inflammation with impaired lymphatic function and different changes in lymphatic vessels in acute and chronic colitis.Am J Transl Res. 2016 Feb 15;8(2):827-41. eCollection 2016. Am J Transl Res. 2016. PMID: 27158372 Free PMC article.
References
-
- Alitalo K. Growth factors controlling angiogenesis and lymphangiogenesis. Ugeskr Laeger 164: 3170–3172, 2002 - PubMed
-
- Alitalo K, Carmeliet P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell 1: 219–227, 2002 - PubMed
-
- Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature 438: 946–953, 2005 - PubMed
-
- Austyn JM, Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol 11: 805–815, 1981 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

