Androgens induce nongenomic stimulation of colonic contractile activity through induction of calcium sensitization and phosphorylation of LC20 and CPI-17
- PMID: 20207835
- PMCID: PMC5417498
- DOI: 10.1210/me.2009-0472
Androgens induce nongenomic stimulation of colonic contractile activity through induction of calcium sensitization and phosphorylation of LC20 and CPI-17
Abstract
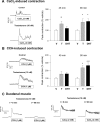
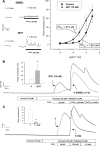
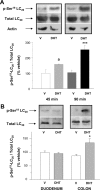
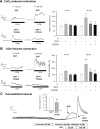
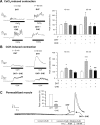
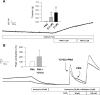
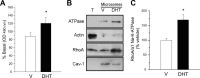
We show that androgens, testosterone and 5alpha-dihydrotestosterone (DHT), acutely (approximately 40 min) provoke the mechanical potentiation of spontaneous and agonist-induced contractile activity in mouse colonic longitudinal smooth muscle. The results using flutamide, finasteride, cycloheximide, and actinomycin D indicate that androgen-induced potentiation is dependent on androgen receptors, requires reduction of testosterone to DHT, and occurs independently of transcriptional and translational events. Using permeabilized colonic smooth muscle preparations, we could demonstrate that mechanical potentiation is entirely due to calcium sensitization of contractile machinery. In addition, DHT (10 nm) increased phosphorylation of both 20-kDa myosin light chain (LC(20)) [regulatory myosin light chain, (MLC)] and CPI-17 (an endogenous inhibitor of MLC phosphatase). Paralleling these findings, inhibition of Rho-associated Rho kinase (ROK) and/or protein kinase C (PKC) with, respectively, Y27632 and chelerythrine, prevented LC(20) phosphorylation and abolished calcium sensitization. In addition, inhibition of ROK prevents CPI-17 phosphorylation, indicating that ROK is located upstream PKC-mediated CPI-17 modulation in the signalling cascade. Additionally, androgens induce a rapid activation of RhoA and its translocation to the plasma membrane to activate ROK. The results demonstrate that androgens induce sensitization of colonic smooth muscle to calcium through activation of ROK, which in turn, activates PKC to induce CPI-17 phosphorylation. Activation of this pathway induces a potent steady stimulation of LC(20) by inhibiting MLC phosphatase and displacing the equilibrium of the regulatory subunit towards its phosphorylated state. This is the first demonstration that colonic smooth muscle is a physiological target for androgen hormones, and that androgens modulate force generation of smooth muscle contractile machinery through nongenomic calcium sensitization pathways.
Figures




Similar articles
-
Androgens differentially potentiate mouse intestinal smooth muscle by nongenomic activation of polyamine synthesis and Rho kinase activation.Endocrinology. 2006 Dec;147(12):5715-29. doi: 10.1210/en.2006-0780. Epub 2006 Aug 31. Endocrinology. 2006. PMID: 16946014
-
Polyamines transduce the nongenomic, androgen-induced calcium sensitization in intestinal smooth muscle.Mol Endocrinol. 2013 Oct;27(10):1603-16. doi: 10.1210/me.2013-1201. Epub 2013 Sep 3. Mol Endocrinol. 2013. PMID: 24002652 Free PMC article.
-
Myosin light chain kinase activation and calcium sensitization in smooth muscle in vivo.Am J Physiol Cell Physiol. 2008 Aug;295(2):C358-64. doi: 10.1152/ajpcell.90645.2007. Epub 2008 Jun 4. Am J Physiol Cell Physiol. 2008. PMID: 18524939 Free PMC article.
-
Androgens are powerful non-genomic inducers of calcium sensitization in visceral smooth muscle.Steroids. 2010 Aug-Sep;75(8-9):533-8. doi: 10.1016/j.steroids.2009.09.012. Epub 2009 Oct 1. Steroids. 2010. PMID: 19800357 Review.
-
The regulation of smooth muscle contractility by zipper-interacting protein kinase.Can J Physiol Pharmacol. 2007 Jan;85(1):79-87. doi: 10.1139/y06-103. Can J Physiol Pharmacol. 2007. PMID: 17487247 Review.
Cited by
-
Calcium Sensitization Mechanisms in Gastrointestinal Smooth Muscles.J Neurogastroenterol Motil. 2016 Apr 30;22(2):213-25. doi: 10.5056/jnm15186. J Neurogastroenterol Motil. 2016. PMID: 26701920 Free PMC article. Review.
-
Regulation of basal LC20 phosphorylation by MYPT1 and CPI-17 in murine gastric antrum, gastric fundus, and proximal colon smooth muscles.Neurogastroenterol Motil. 2011 Oct;23(10):e425-36. doi: 10.1111/j.1365-2982.2011.01769.x. Epub 2011 Aug 24. Neurogastroenterol Motil. 2011. PMID: 21883701 Free PMC article.
-
Translational Perspective on the Role of Testosterone in Sexual Function and Dysfunction.J Sex Med. 2016 Aug;13(8):1183-98. doi: 10.1016/j.jsxm.2016.06.004. J Sex Med. 2016. PMID: 27436075 Free PMC article. Review.
-
Nandrolone decanoate impairs gastrointestinal motility and duodenal morphometry in moderately exercised rats.Braz J Med Biol Res. 2024 Jul 1;57:e13452. doi: 10.1590/1414-431X2024e13452. eCollection 2024. Braz J Med Biol Res. 2024. PMID: 38958368 Free PMC article.
-
The importance of non-nuclear AR signaling in prostate cancer progression and therapeutic resistance.Cell Signal. 2016 May;28(5):348-356. doi: 10.1016/j.cellsig.2016.01.013. Epub 2016 Jan 29. Cell Signal. 2016. PMID: 26829214 Free PMC article. Review.
References
-
- Michels G, Hoppe UC2008. Rapid actions of androgens. Front Neuroendocrinol 29:182–198 - PubMed
-
- Lieberherr M, Grosse B1994. Androgens increase intracellular calcium concentration and inositol 1,4,5-trisphosphate and diacylglycerol formation via a pertussis toxin- sensitive G-protein. J Biol Chem 269:7217–7223 - PubMed
-
- Guo Z, Benten WP, Krücken J, Wunderlich F2002. Nongenomic testosterone calcium signaling. Genotropic actions in androgen receptor-free macrophages. J Biol Chem 277:29600–29607 - PubMed
-
- Benten WP, Lieberherr M, Giese G, Wrehlke C, Stamm O, Sekeris CE, Mossmann H, Wunderlich F1999. Functional testosterone receptors in plasma membranes of T cells. FASEB J 13:123–133 - PubMed

