Long-term follow-up of patients with moderate aplastic anemia and pure red cell aplasia treated with daclizumab
- PMID: 20207845
- PMCID: PMC2833067
- DOI: 10.3324/haematol.2009.013557
Long-term follow-up of patients with moderate aplastic anemia and pure red cell aplasia treated with daclizumab
Abstract
Background: Pure red cell aplasia and moderate aplastic anemia are marrow failure states with an immune pathogenesis. Previously, we described short-term improvements in blood counts in two pilot studies treating moderate aplastic anemia (mAA) and pure red cell aplasia (PRCA) patients with daclizumab, a humanized monoclonal antibody to the interleukin-2 receptor; we now report our long-term experience with a larger cohort of patients.
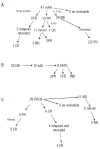
Design and methods: After a median follow-up period of 4.8 years, 19 of 45 (42%) evaluable mAA patients and 10 of 26 (38%) patients with PRCA responded by three months and 2 additional mAA patients responded by six months following administration of the drug.
Results: Seven of 28 (25%) mAA patients achieved long-term packed red blood cell PRBC transfusion independence, and all PRCA responders achieved long-term transfusion PRBC transfusion independence.
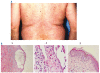
Conclusions: Red cell transfusion-independence prior to treatment in mAA patients predicted response. The only significant adverse treatment-related events were transient rashes and arthralgias. Daclizumab is safe and effective, and produces lengthy remissions in patients with PRCA and mAA.
Figures


Similar articles
-
Brief communication: Successful treatment of pure red-cell aplasia with an anti-interleukin-2 receptor antibody (daclizumab).Ann Intern Med. 2006 Feb 7;144(3):181-5. doi: 10.7326/0003-4819-144-3-200602070-00006. Ann Intern Med. 2006. PMID: 16461962
-
Recombinant humanized anti-IL-2 receptor antibody (daclizumab) produces responses in patients with moderate aplastic anemia.Blood. 2003 Nov 15;102(10):3584-6. doi: 10.1182/blood-2003-04-1032. Epub 2003 Jul 24. Blood. 2003. PMID: 12881307 Clinical Trial.
-
[Clinical study of ciclosporin in patients with aplastic anemia and pure red-cell aplasia].Rinsho Ketsueki. 1995 Mar;36(3):175-84. Rinsho Ketsueki. 1995. PMID: 7783321 Clinical Trial. Japanese.
-
Aplastic anemia and pure red cell aplasia associated with large granular lymphocyte leukemia.Semin Hematol. 2003 Jul;40(3):196-200. doi: 10.1016/s0037-1963(03)00140-9. Semin Hematol. 2003. PMID: 12876668 Review.
-
Anti-Tac (daclizumab, Zenapax) in the treatment of leukemia, autoimmune diseases, and in the prevention of allograft rejection: a 25-year personal odyssey.J Clin Immunol. 2007 Jan;27(1):1-18. doi: 10.1007/s10875-006-9060-0. Epub 2007 Jan 11. J Clin Immunol. 2007. PMID: 17216565 Review.
Cited by
-
Distinctive and common features of moderate aplastic anaemia.Br J Haematol. 2020 Jun;189(5):967-975. doi: 10.1111/bjh.16460. Epub 2020 Jan 31. Br J Haematol. 2020. PMID: 32004386 Free PMC article.
-
Aplastic anemia: immunosuppressive therapy in 2010.Pediatr Rep. 2011 Jun 22;3 Suppl 2(Suppl 2):e7. doi: 10.4081/pr.2011.s2.e7. Pediatr Rep. 2011. PMID: 22053283 Free PMC article.
-
Cutaneous adverse events in multiple sclerosis patients treated with daclizumab.Neurology. 2016 Mar 1;86(9):847-55. doi: 10.1212/WNL.0000000000002417. Epub 2016 Feb 3. Neurology. 2016. PMID: 26843560 Free PMC article. Clinical Trial.
-
Monoclonal gammopathy-associated pure red cell aplasia.Br J Haematol. 2016 Jun;173(6):876-83. doi: 10.1111/bjh.14012. Epub 2016 Mar 21. Br J Haematol. 2016. PMID: 26999424 Free PMC article.
-
The progression risk factors of children with transfusion-independent non-severe aplastic anemia.Int J Hematol. 2013 Feb;97(2):210-5. doi: 10.1007/s12185-013-1263-6. Epub 2013 Jan 30. Int J Hematol. 2013. PMID: 23361447
References
-
- Young NS, Tisdale JF. High-dose cyclophosphamide for treatment of aplastic anemia. Ann Intern Med. 2002;137(6):549–50. - PubMed
-
- Dantal J, Hourmant M, Cantarovich D, Giral M, Blancho G, Dreno B, et al. Effect of long-term immunosuppression in kidney-graft recipients on cancer incidence: randomised comparison of two cyclosporin regimens. Lancet. 1998;351(9103):623–8. - PubMed
-
- Fujishima N, Sawada K, Hirokawa M, Oshimi K, Sugimoto K, Matsuda A, et al. Long-term response and outcome following immunosuppressive therapy in thymoma-associated pure red cell aplasia: a nationwide cohort study in Japan by the PRCA collaborative study group. Haematologica. 2008;93(10):1555–9. - PubMed
-
- Shimamoto T, Tohyama K, Okamoto T, Uchiyama T, Mori H, Tomonaga M, et al. Cyclosporin A therapy for patients with myelodysplastic syndrome: multicenter pilot studies in Japan. Leuk Res. 2003;27(9):783–8. - PubMed
-
- Mor E, Yussim A, Chodoff L, Schwartz ME. New immunosuppressive agents for maintenance therapy in organ transplantation. BioDrugs. 1997;8(6):469–88. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

