Nuclear dynamics, mitosis, and the cytoskeleton during the early stages of colony initiation in Neurospora crassa
- PMID: 20207852
- PMCID: PMC2918927
- DOI: 10.1128/EC.00329-09
Nuclear dynamics, mitosis, and the cytoskeleton during the early stages of colony initiation in Neurospora crassa
Abstract
Neurospora crassa macroconidia form germ tubes that are involved in colony establishment and conidial anastomosis tubes (CATs) that fuse to form interconnected networks of conidial germlings. Nuclear and cytoskeletal behaviors were analyzed in macroconidia, germ tubes, and CATs in strains that expressed fluorescently labeled proteins. Heterokaryons formed by CAT fusion provided a rapid method for the imaging of multiple labeled fusion proteins and minimized the potential risk of overexpression artifacts. Mitosis occurred more slowly in nongerminated macroconidia (1.0 to 1.5 h) than in germ tubes (16 to 20 min). The nucleoporin SON-1 was not released from the nuclear envelope during mitosis, which suggests that N. crassa exhibits a form of "closed mitosis." During CAT homing, nuclei did not enter CATs, and mitosis was arrested. Benomyl treatment showed that CAT induction, homing, fusion, as well as nuclear migration through fused CATs do not require microtubules or mitosis. Three ropy mutants (ro-1, ro-3, and ro-11) defective in the dynein/dynactin microtubule motor were impaired in nuclear positioning, but nuclei still migrated through fused CATs. Latrunculin B treatment, imaging of F-actin in living cells using Lifeact-red fluorescent protein (RFP), and analysis of mutants defective in the Arp2/3 complex demonstrated that actin plays important roles in CAT fusion.
Figures
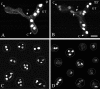
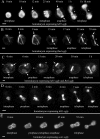


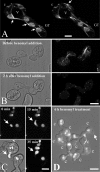


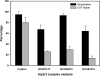


Similar articles
-
F-actin dynamics in Neurospora crassa.Eukaryot Cell. 2010 Apr;9(4):547-57. doi: 10.1128/EC.00253-09. Epub 2010 Feb 5. Eukaryot Cell. 2010. PMID: 20139238 Free PMC article.
-
Microscopic analysis of Neurospora ropy mutants defective in nuclear distribution.Fungal Genet Biol. 1999 Oct;28(1):55-67. doi: 10.1006/fgbi.1999.1160. Fungal Genet Biol. 1999. PMID: 10512672
-
Induction of multiple germ tubes in Neurospora crassa by antitubulin agents.Eur J Cell Biol. 1988 Apr;46(1):68-79. Eur J Cell Biol. 1988. PMID: 2969337
-
[Dynein and dynactin as organizers of the system of cell microtubules].Ontogenez. 2006 Sep-Oct;37(5):323-39. Ontogenez. 2006. PMID: 17066975 Review. Russian.
-
Self-signalling and self-fusion in filamentous fungi.Curr Opin Microbiol. 2009 Dec;12(6):608-15. doi: 10.1016/j.mib.2009.09.008. Epub 2009 Oct 26. Curr Opin Microbiol. 2009. PMID: 19864177 Review.
Cited by
-
F-actin dynamics in Neurospora crassa.Eukaryot Cell. 2010 Apr;9(4):547-57. doi: 10.1128/EC.00253-09. Epub 2010 Feb 5. Eukaryot Cell. 2010. PMID: 20139238 Free PMC article.
-
Syncytial Assembly Lines: Consequences of Multinucleate Cellular Compartments for Fungal Protein Synthesis.Results Probl Cell Differ. 2024;71:159-183. doi: 10.1007/978-3-031-37936-9_9. Results Probl Cell Differ. 2024. PMID: 37996678 Review.
-
Nuclear dynamics during germination, conidiation, and hyphal fusion of Fusarium oxysporum.Eukaryot Cell. 2010 Aug;9(8):1216-24. doi: 10.1128/EC.00040-10. Epub 2010 Jun 11. Eukaryot Cell. 2010. PMID: 20543061 Free PMC article.
-
Internuclear diffusion of histone H1 within cellular compartments of Aspergillus nidulans.PLoS One. 2018 Aug 16;13(8):e0201828. doi: 10.1371/journal.pone.0201828. eCollection 2018. PLoS One. 2018. PMID: 30114268 Free PMC article.
-
Inducible Cell Fusion Permits Use of Competitive Fitness Profiling in the Human Pathogenic Fungus Aspergillus fumigatus.Antimicrob Agents Chemother. 2018 Dec 21;63(1):e01615-18. doi: 10.1128/AAC.01615-18. Print 2019 Jan. Antimicrob Agents Chemother. 2018. PMID: 30397071 Free PMC article.
References
-
- Aist J. R. 2002. Mitosis and motor proteins in the filamentous ascomycete, Nectria haematococca, and some related fungi. Int. Rev. Cytol. 212:239–263 - PubMed
-
- Aist J. R., Morris N. R. 1999. Mitosis in filamentous fungi: how we got where we are. Fungal Genet. Biol. 27:1–25 - PubMed
-
- Araujo-Bazán L., Peñalva M. A., Espeso E. A. 2008. Preferential localization of the endocytic internalization machinery to hyphal tips underlies polarization of the actin cytoskeleton in Aspergillus nidulans. Mol. Microbiol. 67:891–905 - PubMed
-
- Araujo-Palomares C. L., Castro-Longoria E., Riquelme M. 2007. Ontogeny of the Spitzenkörper in germlings of Neurospora crassa. Fungal Genet. Biol. 44:492–503 - PubMed
-
- Asakura T., Sasaki T., Nagano F., Satoh A., Obaishi H., Nishioka H., Imamura H., Hotta K., Tanaka K., Nakanishi H., Takai Y. 1998. Isolation and characterization of a novel actin filament-binding protein from Saccharomyces cerevisiae. Oncogene 16:121–130 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

