Clinical efficacy comparison of Saccharomyces boulardii and yogurt fluid in acute non-bloody diarrhea in children: a randomized, controlled, open label study
- PMID: 20207879
- PMCID: PMC2829915
- DOI: 10.4269/ajtmh.2010.09-0529
Clinical efficacy comparison of Saccharomyces boulardii and yogurt fluid in acute non-bloody diarrhea in children: a randomized, controlled, open label study
Abstract
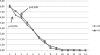
The purpose of this trial is to evaluate the clinical efficacy and cost/effectiveness of Saccharomyces boulardii compared with yogurt fluid (YF) in acute non-bloody diarrhea in children. This randomized, prospective open-label clinical trial includes 55 children (36 boys, 19 girls; mean age 21.2 +/- 28.2 months). Group A (N = 28) received lyophilized S. boulardii and group B (N = 27) received YF. The duration of diarrhea was shorter with S. boulardii but the hospital stay was reduced with YF, although these differences were not significant. However, diarrhea had resolved in significantly more children on day 3 in the S. boulardii group (48.5% versus 25.5%; P < 0.05). In outpatient cases, yogurt treatment was cheaper than S. boulardii whereas in hospitalized patients, treatment cost was similar. In conclusion, the effect of daily freshly prepared YF was comparable to S. boulardii in the treatment of acute non-bloody diarrhea in children. The duration of diarrhea was shorter in the S. boulardii group, expressed as a significantly higher number of patients with normal stools on day 3.
Figures


References
-
- Sazawal S, Dhingra U, Dhingra P, Hiremath G, Kumar J, Sarkar A, Menon VP, Black RE. Efficacy of probiotics in prevention of acute diarrhoea: a meta-analysis of masked, randomised, placebo-controlled trials. Lancet Infect Dis. 2006;6:374–382. - PubMed
-
- Guarino A, Albano F, Ashkenazi S, Gendrel D, Hoekstra JH, Shamir R, Szajewska H. European Society for Paediatric Gastroenterology, Hepatology, and Nutrition/European Society for Paediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe. J Pediatr Gastroenterol Nutr. 2008;46(Suppl 2):S81–S122. - PubMed
-
- Vandenplas Y, Salvatore S, Viera M, Devreker T, Hauser B. Probiotics in infectious diarrhoea in children: are they indicated? Eur J Pediatr. 2007;166:1211–1218. - PubMed
-
- Vandenplas Y, Brunser O, Szajewska H.2009Saccharomyces boulardii in childhood. Eur J Pediatr 168253–265. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

