Forward operation of adenine nucleotide translocase during F0F1-ATPase reversal: critical role of matrix substrate-level phosphorylation
- PMID: 20207940
- PMCID: PMC2887268
- DOI: 10.1096/fj.09-149898
Forward operation of adenine nucleotide translocase during F0F1-ATPase reversal: critical role of matrix substrate-level phosphorylation
Abstract
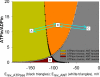
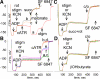
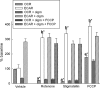
In pathological conditions, F(0)F(1)-ATPase hydrolyzes ATP in an attempt to maintain mitochondrial membrane potential. Using thermodynamic assumptions and computer modeling, we established that mitochondrial membrane potential can be more negative than the reversal potential of the adenine nucleotide translocase (ANT) but more positive than that of the F(0)F(1)-ATPase. Experiments on isolated mitochondria demonstrated that, when the electron transport chain is compromised, the F(0)F(1)-ATPase reverses, and the membrane potential is maintained as long as matrix substrate-level phosphorylation is functional, without a concomitant reversal of the ANT. Consistently, no cytosolic ATP consumption was observed using plasmalemmal K(ATP) channels as cytosolic ATP biosensors in cultured neurons, in which their in situ mitochondria were compromised by respiratory chain inhibitors. This finding was further corroborated by quantitative measurements of mitochondrial membrane potential, oxygen consumption, and extracellular acidification rates, indicating nonreversal of ANT of compromised in situ neuronal and astrocytic mitochondria; and by bioluminescence ATP measurements in COS-7 cells transfected with cytosolic- or nuclear-targeted luciferases and treated with mitochondrial respiratory chain inhibitors in the presence of glycolytic plus mitochondrial vs. only mitochondrial substrates. Our findings imply the possibility of a rescue mechanism that is protecting against cytosolic/nuclear ATP depletion under pathological conditions involving impaired respiration. This mechanism comes into play when mitochondria respire on substrates that support matrix substrate-level phosphorylation.
Figures


References
-
- Rouslin W, Erickson J L, Solaro R J. Effects of oligomycin and acidosis on rates of ATP depletion in ischemic heart muscle. Am J Physiol. 1986;250:H503–H508. - PubMed
-
- McMillin J B, Pauly D F. Control of mitochondrial respiration in muscle. Mol Cell Biochem. 1988;81:121–129. - PubMed
-
- Petronilli V, Azzone G F, Pietrobon D. Analysis of mechanisms of free-energy coupling and uncoupling by inhibitor titrations: theory, computer modeling and experiments. Biochim Biophys Acta. 1988;932:306–324. - PubMed
-
- Rouslin W, Broge C W, Grupp I L. ATP depletion and mitochondrial functional loss during ischemia in slow and fast heart-rate hearts. Am J Physiol. 1990;259:H1759–H1766. - PubMed
-
- Wisniewski E, Kunz W S, Gellerich F N. Phosphate affects the distribution of flux control among the enzymes of oxidative phosphorylation in rat skeletal muscle mitochondria. J Biol Chem. 1993;268:9343–9346. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

