The activated state of a sodium channel voltage sensor in a membrane environment
- PMID: 20207950
- PMCID: PMC2851821
- DOI: 10.1073/pnas.0914109107
The activated state of a sodium channel voltage sensor in a membrane environment
Abstract
Direct structural insights on the fundamental mechanisms of permeation, selectivity, and gating remain unavailable for the Na(+) and Ca(2+) channel families. Here, we report the spectroscopic structural characterization of the isolated Voltage-Sensor Domain (VSD) of the prokaryotic Na(+) channel NaChBac in a lipid bilayer. Site-directed spin-labeling and EPR spectroscopy were carried out for 118 mutants covering all of the VSD. EPR environmental data were used to unambiguously assign the secondary structure elements, define membrane insertion limits, and evaluate the activated conformation of the isolated-VSD in the membrane using restrain-driven molecular dynamics simulations. The overall three-dimensional fold of the NaChBac-VSD closely mirrors those seen in KvAP, Kv1.2, Kv1.2-2.1 chimera, and MlotiK1. However, in comparison to the membrane-embedded KvAP-VSD, the structural dynamics of the NaChBac-VSD reveals a much tighter helix packing, with subtle differences in the local environment of the gating charges and their interaction with the rest of the protein. Using cell complementation assays we show that the NaChBac-VSD can provide a conduit to the transport of ions in the resting or "down" conformation, a feature consistent with our EPR water accessibility measurements in the activated or "up" conformation. These results suggest that the overall architecture of VSD's is remarkably conserved among K(+) and Na(+) channels and that pathways for gating-pore currents may be intrinsic to most voltage-sensors. Cell complementation assays also provide information about the putative location of the gating charges in the "down/resting" state and hence a glimpse of the extent of conformational changes during activation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
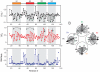
 (black), O2 accessibility ΠO2 (red) and NiEdda accessibility ΠNiEdda (blue). The gray regions represent the putative TM segments from the hydropathy plot. Green asterisks denote highly conserved residues with VSDs. Bent arrows point to the increase in ΠO2 towards the center of the bilayer in S1 and S2 and straight arrows indicate a break in helix periodicity in S3 and S4. (B) Helical wheel representation of ΠO2 superimposed in a polar coordinate representation. The relative orientation of the helices can be predicated based on the direction of the resultant vector, which points towards the lipid facing phase of the helix. The shaded area within the dashed lines highlights the degree of eccentricity for the complete set of accessibility data relative to the maximal accessibility vector.
(black), O2 accessibility ΠO2 (red) and NiEdda accessibility ΠNiEdda (blue). The gray regions represent the putative TM segments from the hydropathy plot. Green asterisks denote highly conserved residues with VSDs. Bent arrows point to the increase in ΠO2 towards the center of the bilayer in S1 and S2 and straight arrows indicate a break in helix periodicity in S3 and S4. (B) Helical wheel representation of ΠO2 superimposed in a polar coordinate representation. The relative orientation of the helices can be predicated based on the direction of the resultant vector, which points towards the lipid facing phase of the helix. The shaded area within the dashed lines highlights the degree of eccentricity for the complete set of accessibility data relative to the maximal accessibility vector.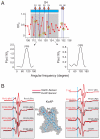
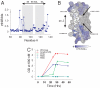

 ), O2 accessibility parameter (ΠO2) and NiEdda accessibility parameters (ΠNiEdda ) are mapped on to a surface representation of the model.
), O2 accessibility parameter (ΠO2) and NiEdda accessibility parameters (ΠNiEdda ) are mapped on to a surface representation of the model.
Similar articles
-
EPR Studies of Gating Mechanisms in Ion Channels.Methods Enzymol. 2015;557:279-306. doi: 10.1016/bs.mie.2014.12.030. Epub 2015 Mar 24. Methods Enzymol. 2015. PMID: 25950970 Free PMC article. Review.
-
Structural characterization of the voltage-sensor domain and voltage-gated K+-channel proteins vectorially oriented within a single bilayer membrane at the solid/vapor and solid/liquid interfaces via neutron interferometry.Langmuir. 2012 Jul 17;28(28):10504-20. doi: 10.1021/la301219z. Epub 2012 Jun 29. Langmuir. 2012. PMID: 22686684 Free PMC article.
-
Distance measurements reveal a common topology of prokaryotic voltage-gated ion channels in the lipid bilayer.Proc Natl Acad Sci U S A. 2006 Oct 24;103(43):15865-70. doi: 10.1073/pnas.0607532103. Epub 2006 Oct 16. Proc Natl Acad Sci U S A. 2006. PMID: 17043236 Free PMC article.
-
Structural dynamics of an isolated voltage-sensor domain in a lipid bilayer.Structure. 2008 Mar;16(3):398-409. doi: 10.1016/j.str.2007.12.015. Structure. 2008. PMID: 18334215 Free PMC article.
-
Omega currents in voltage-gated ion channels: what can we learn from uncovering the voltage-sensing mechanism using MD simulations?Acc Chem Res. 2013 Dec 17;46(12):2755-62. doi: 10.1021/ar300290u. Epub 2013 May 22. Acc Chem Res. 2013. PMID: 23697886 Review.
Cited by
-
Mechanism of electromechanical coupling in voltage-gated potassium channels.Front Pharmacol. 2012 Sep 12;3:166. doi: 10.3389/fphar.2012.00166. eCollection 2012. Front Pharmacol. 2012. PMID: 22988442 Free PMC article.
-
EPR Studies of Gating Mechanisms in Ion Channels.Methods Enzymol. 2015;557:279-306. doi: 10.1016/bs.mie.2014.12.030. Epub 2015 Mar 24. Methods Enzymol. 2015. PMID: 25950970 Free PMC article. Review.
-
S1-S3 counter charges in the voltage sensor module of a mammalian sodium channel regulate fast inactivation.J Gen Physiol. 2013 May;141(5):601-18. doi: 10.1085/jgp.201210935. Epub 2013 Apr 15. J Gen Physiol. 2013. PMID: 23589580 Free PMC article.
-
Influences of membrane mimetic environments on membrane protein structures.Annu Rev Biophys. 2013;42:361-92. doi: 10.1146/annurev-biophys-083012-130326. Epub 2013 Mar 1. Annu Rev Biophys. 2013. PMID: 23451886 Free PMC article. Review.
-
Structural refinement of the hERG1 pore and voltage-sensing domains with ROSETTA-membrane and molecular dynamics simulations.Proteins. 2010 Nov 1;78(14):2922-34. doi: 10.1002/prot.22815. Proteins. 2010. PMID: 20740484 Free PMC article.
References
-
- Ren D, et al. A prokaryotic voltage-gated sodium channel. Science. 2001;294(5550):2372–2375. - PubMed
-
- Chahine M, Pilote S, Pouliot V, Takami H, Sato C. Role of arginine residues on the S4 segment of the Bacillus halodurans Na+ channel in voltage-sensing. J Membr Biol. 2004;201(1):9–24. - PubMed
-
- Lu Z, Klem AM, Ramu Y. Ion conduction pore is conserved among potassium channels. Nature. 2001;413(6858):809–813. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

