The role of TLR4 in photoreceptor {alpha}a crystallin upregulation during early experimental autoimmune uveitis
- PMID: 20207969
- PMCID: PMC2904017
- DOI: 10.1167/iovs.09-4575
The role of TLR4 in photoreceptor {alpha}a crystallin upregulation during early experimental autoimmune uveitis
Abstract
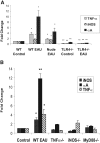
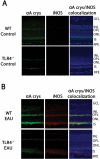
Purpose. Previous studies indicate that the upregulation of alphaA crystallin prevents photoreceptor mitochondrial oxidative stress-mediated apoptosis in experimental autoimmune uveitis (EAU). In this study, the role of TLR4 was investigated in the upregulation of alphaA crystallin in the retinas of animals with EAU. Methods. TLR4(-/-), iNOS(-/-), TNF-alpha(-/-), MyD88(-/-), wild-type (WT) control (C57BL/6), and nude mice (B6.Cg-Foxn1(nu)) were immunized with IRBP mixed with complete Freund's adjuvant; eyes were enucleated on day 7 after immunization. Real-time polymerase chain reaction was first used to detect upregulated inflammatory cytokines and alphaA crystallin in retinas with EAU; confirmed with Western blot analysis, and the site of upregulation was localized by immunohistochemistry. Oxidative stress was localized using 8-OHdG, and TUNEL staining was used to detect apoptosis. Results. In early EAU, increased expression of TNF-alpha, iNOS, and alphaA crystallin genes were detected in the retinas of WT mice, whereas such upregulation was absent in TLR4-deficient mice (P < 0.001). alphaA Crystallin was not elevated in MyD88(-/-), TNF-alpha(-/-), and iNOS(-/-) mice with EAU. Immunostaining revealed TNF-alpha, iNOS, and alphaA crystallin localization in the photoreceptor inner segments and outer plexiform layer in the WT controls with EAU; but such staining was absent in TLR4-deficient mice with EAU. 8-OHdG staining showed oxidative stress in the photoreceptors in WT mice with EAU and there was no apoptosis. Conclusions. TLR4 plays an important role in the upregulation of alphaA crystallin through the interaction of MyD88 and the subsequent generation of TNF-alpha and iNOS in the EAU retina. Such crystallin upregulation may prevent oxidative stress-mediated apoptosis of photoreceptors in uveitis.
Figures




Similar articles
-
Elevated retina-specific expression of the small heat shock protein, alphaA-crystallin, is associated with photoreceptor protection in experimental uveitis.Invest Ophthalmol Vis Sci. 2008 Mar;49(3):1161-71. doi: 10.1167/iovs.07-1259. Invest Ophthalmol Vis Sci. 2008. PMID: 18326745
-
Photoreceptor mitochondrial oxidative stress in early experimental autoimmune uveoretinitis.Br J Ophthalmol. 2007 Apr;91(4):531-7. doi: 10.1136/bjo.2006.101576. Epub 2006 Oct 11. Br J Ophthalmol. 2007. PMID: 17035279 Free PMC article.
-
Mitochondrial proteomics in experimental autoimmune uveitis oxidative stress.Invest Ophthalmol Vis Sci. 2009 Dec;50(12):5559-66. doi: 10.1167/iovs.08-2842. Epub 2009 Jul 2. Invest Ophthalmol Vis Sci. 2009. PMID: 19578012 Free PMC article.
-
The Role of αA-Crystallin in Experimental Autoimmune Uveitis.Curr Mol Med. 2015;15(6):558-64. doi: 10.2174/1566524015666150731101146. Curr Mol Med. 2015. PMID: 26238368 Review.
-
Photoreceptor mitochondrial oxidative stress in experimental autoimmune uveitis.Ophthalmic Res. 2008;40(3-4):160-4. doi: 10.1159/000119869. Epub 2008 Apr 18. Ophthalmic Res. 2008. PMID: 18421232 Review.
Cited by
-
Therapeutic potential of α-crystallin.Biochim Biophys Acta. 2016 Jan;1860(1 Pt B):252-7. doi: 10.1016/j.bbagen.2015.03.012. Epub 2015 Apr 1. Biochim Biophys Acta. 2016. PMID: 25840354 Free PMC article. Review.
-
Neuroglobin protection in retinal ischemia.Invest Ophthalmol Vis Sci. 2012 Feb 13;53(2):704-11. doi: 10.1167/iovs.11-7408. Print 2012 Feb. Invest Ophthalmol Vis Sci. 2012. PMID: 22167093 Free PMC article.
-
Role of ATP-Small Heat Shock Protein Interaction in Human Diseases.Front Mol Biosci. 2022 Feb 16;9:844826. doi: 10.3389/fmolb.2022.844826. eCollection 2022. Front Mol Biosci. 2022. PMID: 35252358 Free PMC article. Review.
-
HSPB4/CRYAA Protect Photoreceptors during Retinal Detachment in Part through FAIM2 Regulation.Neurol Int. 2024 Aug 26;16(5):905-917. doi: 10.3390/neurolint16050068. Neurol Int. 2024. PMID: 39311341 Free PMC article.
-
Local Myeloid-Derived Suppressor Cells Impair Progression of Experimental Autoimmune Uveitis by Alleviating Oxidative Stress and Inflammation.Invest Ophthalmol Vis Sci. 2023 Oct 3;64(13):39. doi: 10.1167/iovs.64.13.39. Invest Ophthalmol Vis Sci. 2023. PMID: 37878302 Free PMC article.
References
-
- Arocker-Mettinger E, Grabner G. Uveitis: a status assessment. Fortschr Ophthalmol 1990;87(suppl):S110–S117 - PubMed
-
- Nussenblatt RB. Bench to bedside: new approaches to the immunotherapy of uveitic disease. Int Rev Immunol 2002;21:273–289 - PubMed
-
- Agarwal RK, Caspi RR. Rodent models of experimental autoimmune uveitis. Methods Mol Med 2004;102:395–419 - PubMed
-
- Forrester JV, Huitinga I, Lumsden L, Dijkstra CD. Marrow-derived activated macrophages are required during the effector phase of experimental autoimmune uveoretinitis in rats. Curr Eye Res 1998;17:426–437 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

