Regulatory T cells inhibit T cell proliferation and decrease demyelination in mice chronically infected with a coronavirus
- PMID: 20208000
- PMCID: PMC2851486
- DOI: 10.4049/jimmunol.0903918
Regulatory T cells inhibit T cell proliferation and decrease demyelination in mice chronically infected with a coronavirus
Abstract
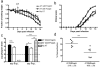
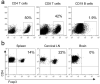
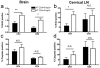
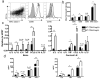
Mice infected with the neurotropic JHM strain of mouse hepatitis virus (JHMV) develop acute and chronic demyelinating diseases with histopathological similarities to multiple sclerosis. The process of demyelination is largely immune-mediated, as immunodeficient mice (RAG1(-/-) mice) do not develop demyelination upon infection; however, demyelination develops if these mice are reconstituted with either JHMV-immune CD4 or CD8 T cells. Because myelin destruction is a consequence of the inflammatory response associated with virus clearance, we reasoned that decreasing the amount of inflammation would diminish clinical disease and demyelination. Given that regulatory T cells (Tregs) have potent anti-inflammatory effects, we adoptively transferred Tregs into infected C57BL/6 and RAG1(-/-) mice. In both instances, transfer of Tregs decreased weight loss, clinical scores, and demyelination. Transferred Tregs were not detected in the CNS of infected RAG1(-/-) mice, but rather appeared to mediate their effects in the draining cervical lymph nodes. We show that Tregs dampen the inflammatory response mediated by transferred JHMV-immune splenocytes in infected RAG1(-/-) mice by decreasing T cell proliferation, dendritic cell activation, and proinflammatory cytokine/chemokine production, without inducing apoptosis. By extension, decreasing inflammation, whether by Treg transfer or by otherwise enhancing the anti-inflammatory milieu, could contribute to improved clinical outcomes in patients with virus-induced demyelination.
Figures





References
-
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. - PubMed
-
- Feuerer M, Hill JA, Mathis D, Benoist C. Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nat Immunol. 2009;10:689–695. - PubMed
-
- Shevach EM. From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity. 2006;25:195–201. - PubMed
-
- Robertson SJ, Messer RJ, Carmody AB, Hasenkrug KJ. In vitro suppression of CD8+ T cell function by Friend virus-induced regulatory T cells. J Immunol. 2006;176:3342–3349. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

