Cell surface relocalization of the endoplasmic reticulum chaperone and unfolded protein response regulator GRP78/BiP
- PMID: 20208072
- PMCID: PMC2865300
- DOI: 10.1074/jbc.M109.087445
Cell surface relocalization of the endoplasmic reticulum chaperone and unfolded protein response regulator GRP78/BiP
Abstract
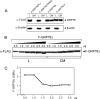
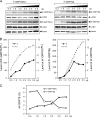
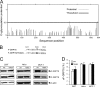
The recent discovery that GRP78/BiP, a typical endoplasmic reticulum (ER) lumenal chaperone, can be expressed on the cell surface, interacting with an increasing repertoire of surface proteins and acting as receptor in signaling pathways, represents a paradigm shift in its biological function. However, the mechanism of GRP78 trafficking from the ER to the cell surface is not well understood. Using a combination of cellular, biochemical, and mutational approaches, we tested multiple hypotheses. Here we report that ER stress actively promotes GRP78 localization on the cell surface, whereas ectopic expression of GRP78 is also able to cause cell surface relocation in the absence of ER stress. Moreover, deletion of the C-terminal ER retention motif in GRP78 alters its cell surface presentation in a dose-dependent manner; however, mutation of the putative O-linked glycosylation site Thr(648) of human GRP78 is without effect. We also identified the exposure of multiple domains of GRP78 on the cell surface and determined that binding of extracellular GRP78 to the cell surface is unlikely. A new topology model for cell surface GRP78 is presented.
Figures


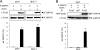




Similar articles
-
Stress-induced translocation of the endoplasmic reticulum chaperone GRP78/BiP and its impact on human disease and therapy.Proc Natl Acad Sci U S A. 2025 Jul 29;122(30):e2412246122. doi: 10.1073/pnas.2412246122. Epub 2025 Jul 23. Proc Natl Acad Sci U S A. 2025. PMID: 40699920 Free PMC article.
-
The BiP molecular chaperone plays multiple roles during the biogenesis of torsinA, an AAA+ ATPase associated with the neurological disease early-onset torsion dystonia.J Biol Chem. 2014 May 2;289(18):12727-47. doi: 10.1074/jbc.M113.529123. Epub 2014 Mar 13. J Biol Chem. 2014. PMID: 24627482 Free PMC article.
-
The unfolded protein response regulator GRP78/BiP is required for endoplasmic reticulum integrity and stress-induced autophagy in mammalian cells.Cell Death Differ. 2008 Sep;15(9):1460-71. doi: 10.1038/cdd.2008.81. Epub 2008 Jun 13. Cell Death Differ. 2008. PMID: 18551133 Free PMC article.
-
The endoplasmic reticulum (ER) chaperone BiP is a master regulator of ER functions: Getting by with a little help from ERdj friends.J Biol Chem. 2019 Feb 8;294(6):2098-2108. doi: 10.1074/jbc.REV118.002804. Epub 2018 Dec 18. J Biol Chem. 2019. PMID: 30563838 Free PMC article. Review.
-
GRP78 in lung cancer.J Transl Med. 2021 Mar 21;19(1):118. doi: 10.1186/s12967-021-02786-6. J Transl Med. 2021. PMID: 33743739 Free PMC article. Review.
Cited by
-
Identification of potential tumor differentiation factor (TDF) receptor from steroid-responsive and steroid-resistant breast cancer cells.J Biol Chem. 2012 Jan 13;287(3):1719-33. doi: 10.1074/jbc.M111.284091. Epub 2011 Nov 30. J Biol Chem. 2012. PMID: 22130669 Free PMC article.
-
The natural human IgM antibody PAT-SM6 induces apoptosis in primary human multiple myeloma cells by targeting heat shock protein GRP78.PLoS One. 2013 May 7;8(5):e63414. doi: 10.1371/journal.pone.0063414. Print 2013. PLoS One. 2013. PMID: 23667612 Free PMC article.
-
Suppression of head and neck cancer cell survival and cisplatin resistance by GRP78 small molecule inhibitor YUM70.Front Oncol. 2023 Jan 11;12:1044699. doi: 10.3389/fonc.2022.1044699. eCollection 2022. Front Oncol. 2023. PMID: 36713577 Free PMC article.
-
HPMA copolymer-aminohexylgeldanamycin conjugates targeting cell surface expressed GRP78 in prostate cancer.Pharm Res. 2010 Dec;27(12):2683-93. doi: 10.1007/s11095-010-0267-7. Epub 2010 Sep 16. Pharm Res. 2010. PMID: 20845065
-
Unfolded Protein Response as a Therapeutic Target in Cardiovascular Disease.Curr Top Med Chem. 2019;19(21):1902-1917. doi: 10.2174/1568026619666190521093049. Curr Top Med Chem. 2019. PMID: 31109279 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

