Structural implications of a G170R mutation of alanine:glyoxylate aminotransferase that is associated with peroxisome-to-mitochondrion mistargeting
- PMID: 20208150
- PMCID: PMC2833026
- DOI: 10.1107/S1744309109054645
Structural implications of a G170R mutation of alanine:glyoxylate aminotransferase that is associated with peroxisome-to-mitochondrion mistargeting
Abstract
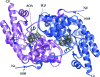
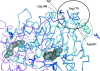
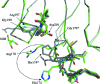
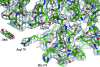
In a subset of patients with the hereditary kidney-stone disease primary hyperoxaluria type 1 (PH1), the liver-specific enzyme alanine:glyoxylate aminotransferase (AGT) is mistargeted from peroxisomes to mitochondria. This is a consequence of the combined presence of the common P11L polymorphism and a disease-specific G170R mutation. In this paper, the crystal structure of mutant human AGT containing the G170R replacement determined at a resolution of 2.6 A is reported. The crystal structure of AGT consists of an intimate dimer in which an extended N-terminal segment of 21 amino acids from one subunit wraps as an elongated irregular coil around the outside of the crystallographic symmetry-related subunit. In addition to the N-terminal segment, the monomer structure contains a large domain of 261 amino acids and a small C-terminal domain of 110 amino acids. Comparison of the mutant AGT structure and that of wild-type normal AGT shows that the two structures are almost identical, with a backbone-atom r.m.s. deviation of 0.34 A. However, evidence of significant local structural changes in the vicinity of the G170R mutation might be linked to the apparent decrease in protein stability.
Figures
Similar articles
-
Alanine:glyoxylate aminotransferase peroxisome-to-mitochondrion mistargeting in human hereditary kidney stone disease.Biochim Biophys Acta. 2003 Apr 11;1647(1-2):70-5. doi: 10.1016/s1570-9639(03)00055-4. Biochim Biophys Acta. 2003. PMID: 12686111 Review.
-
Human liver peroxisomal alanine:glyoxylate aminotransferase: Different stability under chemical stress of the major allele, the minor allele, and its pathogenic G170R variant.Biochimie. 2010 Dec;92(12):1801-11. doi: 10.1016/j.biochi.2010.08.005. Epub 2010 Aug 14. Biochimie. 2010. PMID: 20713123
-
Functional synergism between the most common polymorphism in human alanine:glyoxylate aminotransferase and four of the most common disease-causing mutations.J Biol Chem. 2000 Nov 17;275(46):36415-22. doi: 10.1074/jbc.M006693200. J Biol Chem. 2000. PMID: 10960483
-
Four of the most common mutations in primary hyperoxaluria type 1 unmask the cryptic mitochondrial targeting sequence of alanine:glyoxylate aminotransferase encoded by the polymorphic minor allele.J Biol Chem. 2013 Jan 25;288(4):2475-84. doi: 10.1074/jbc.M112.432617. Epub 2012 Dec 10. J Biol Chem. 2013. PMID: 23229545 Free PMC article.
-
Primary hyperoxaluria type 1: AGT mistargeting highlights the fundamental differences between the peroxisomal and mitochondrial protein import pathways.Biochim Biophys Acta. 2006 Dec;1763(12):1776-84. doi: 10.1016/j.bbamcr.2006.08.021. Epub 2006 Aug 24. Biochim Biophys Acta. 2006. PMID: 17027096 Review.
Cited by
-
Structural dynamics shape the fitness window of alanine:glyoxylate aminotransferase.Protein Sci. 2022 May;31(5):e4303. doi: 10.1002/pro.4303. Protein Sci. 2022. PMID: 35481644 Free PMC article.
-
Functional analysis of amino acid substitutions within human AGT1 in a cell-based platform to support the diagnosis of primary hyperoxaluria type 1.J Biol Chem. 2025 Jul 17;301(8):110494. doi: 10.1016/j.jbc.2025.110494. Online ahead of print. J Biol Chem. 2025. PMID: 40683450 Free PMC article.
-
Ras-activated RSK1 phosphorylates EBP50 to regulate its nuclear localization and promote cell proliferation.Oncotarget. 2016 Mar 1;7(9):10283-96. doi: 10.18632/oncotarget.7184. Oncotarget. 2016. PMID: 26862730 Free PMC article.
-
Molecular requirements for peroxisomal targeting of alanine-glyoxylate aminotransferase as an essential determinant in primary hyperoxaluria type 1.PLoS Biol. 2012;10(4):e1001309. doi: 10.1371/journal.pbio.1001309. Epub 2012 Apr 17. PLoS Biol. 2012. PMID: 22529745 Free PMC article.
-
The role of protein denaturation energetics and molecular chaperones in the aggregation and mistargeting of mutants causing primary hyperoxaluria type I.PLoS One. 2013 Aug 27;8(8):e71963. doi: 10.1371/journal.pone.0071963. eCollection 2013. PLoS One. 2013. PMID: 24205397 Free PMC article.
References
-
- Brünger, A. T., Adams, P. D., Clore, G. M., DeLano, W. L., Gros, P., Grosse-Kunstleve, R. W., Jiang, J.-S., Kuszewski, J., Nilges, M., Pannu, N. S., Read, R. J., Rice, L. M., Simonson, T. & Warren, G. L. (1998). Acta Cryst. D54, 905–921. - PubMed
-
- Cohen, F. E. & Kelly, J. W. (2003). Nature (London), 426, 905–909. - PubMed
-
- Collaborative Computational Project, Number 4 (1994). Acta Cryst. D50, 760–763. - PubMed
-
- Danpure, C. J. (2001). The Molecular and Metabolic Bases of Inherited Disease, edited by C. R. Scriver, A. L. Beaudet, W. S. Sly, D. Valle, B. Childs, K. W. Kinzler & B. Vogelstein, pp. 3323–3367. New York: McGraw–Hill.
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Miscellaneous

