A triclinic crystal form of Escherichia coli 4-diphosphocytidyl-2C-methyl-D-erythritol kinase and reassessment of the quaternary structure
- PMID: 20208151
- PMCID: PMC2833027
- DOI: 10.1107/S1744309109054591
A triclinic crystal form of Escherichia coli 4-diphosphocytidyl-2C-methyl-D-erythritol kinase and reassessment of the quaternary structure
Abstract
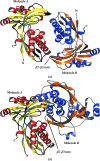
4-Diphosphocytidyl-2C-methyl-D-erythritol kinase (IspE; EC 2.7.1.148) contributes to the 1-deoxy-D-xylulose 5-phosphate or mevalonate-independent biosynthetic pathway that produces the isomers isopentenyl diphosphate and dimethylallyl diphosphate. These five-carbon compounds are the fundamental building blocks for the biosynthesis of isoprenoids. The mevalonate-independent pathway does not occur in humans, but is present and has been shown to be essential in many dangerous pathogens, i.e. Plasmodium species, which cause malaria, and gram-negative bacteria. Thus, the enzymes involved in this pathway have attracted attention as potential drug targets. IspE produces 4-diphosphosphocytidyl-2C-methyl-D-erythritol 2-phosphate by ATP-dependent phosphorylation of 4-diphosphocytidyl-2C-methyl-D-erythritol. A triclinic crystal structure of the Escherichia coli IspE-ADP complex with two molecules in the asymmetric unit was determined at 2 A resolution and compared with a monoclinic crystal form of a ternary complex of E. coli IspE also with two molecules in the asymmetric unit. The molecular packing is different in the two forms. In the asymmetric unit of the triclinic crystal form the substrate-binding sites of IspE are occluded by structural elements of the partner, suggesting that the ;triclinic dimer' is an artefact of the crystal lattice. The surface area of interaction in the triclinic form is almost double that observed in the monoclinic form, implying that the dimeric assembly in the monoclinic form may also be an artifact of crystallization.
Figures



Similar articles
-
Biosynthesis of isoprenoids: crystal structure of 4-diphosphocytidyl-2C-methyl-D-erythritol kinase.Proc Natl Acad Sci U S A. 2003 Aug 5;100(16):9173-8. doi: 10.1073/pnas.1533425100. Epub 2003 Jul 23. Proc Natl Acad Sci U S A. 2003. PMID: 12878729 Free PMC article.
-
Characterization of Aquifex aeolicus 4-diphosphocytidyl-2C-methyl-d-erythritol kinase - ligand recognition in a template for antimicrobial drug discovery.FEBS J. 2008 Jun;275(11):2779-94. doi: 10.1111/j.1742-4658.2008.06418.x. Epub 2008 Apr 16. FEBS J. 2008. PMID: 18422643 Free PMC article.
-
Crystal structure of 4-diphosphocytidyl-2-C-methyl-D-erythritol kinase (IspE) from Mycobacterium tuberculosis.FASEB J. 2011 May;25(5):1577-84. doi: 10.1096/fj.10-175786. Epub 2011 Jan 31. FASEB J. 2011. PMID: 21282208
-
Development of inhibitors of the 2C-methyl-D-erythritol 4-phosphate (MEP) pathway enzymes as potential anti-infective agents.J Med Chem. 2014 Dec 11;57(23):9740-63. doi: 10.1021/jm5010978. Epub 2014 Sep 25. J Med Chem. 2014. PMID: 25210872 Review.
-
Isoprenoid biosynthesis via 1-deoxy-D-xylulose 5-phosphate/2-C-methyl-D-erythritol 4-phosphate (DOXP/MEP) pathway.Acta Biochim Pol. 2001;48(3):663-72. Acta Biochim Pol. 2001. PMID: 11833775 Review.
Cited by
-
Biochemistry of the non-mevalonate isoprenoid pathway.Cell Mol Life Sci. 2011 Dec;68(23):3797-814. doi: 10.1007/s00018-011-0753-z. Epub 2011 Jul 9. Cell Mol Life Sci. 2011. PMID: 21744068 Free PMC article. Review.
-
Positioning Bacillus subtilis as terpenoid cell factory.J Appl Microbiol. 2021 Jun;130(6):1839-1856. doi: 10.1111/jam.14904. Epub 2020 Nov 20. J Appl Microbiol. 2021. PMID: 33098223 Free PMC article. Review.
-
Isoprenoid precursor biosynthesis offers potential targets for drug discovery against diseases caused by apicomplexan parasites.Curr Top Med Chem. 2011;11(16):2048-59. doi: 10.2174/156802611796575867. Curr Top Med Chem. 2011. PMID: 21619509 Free PMC article. Review.
References
-
- Byres, E., Alphey, M. S., Smith, T. K. & Hunter, W. N. (2007). J. Mol. Biol.371, 540–553. - PubMed
-
- Cheek, S., Zhang, H. & Grishin, N. V. (2002). J. Mol. Biol.320, 855–881. - PubMed
-
- Crane, C. M., Hirsch, A. K., Alphey, M. S., Sgraja, T., Lauw, S., Illarionova, V., Rohdich, F., Eisenreich, W., Hunter, W. N., Bacher, A. & Diederich, F. (2008). Chem. Med. Chem.3, 91–101. - PubMed
-
- Cruickshank, D. W. J. (1999). Acta Cryst. D55, 583–601. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

