Transcription-independent ARF regulation in oncogenic stress-mediated p53 responses
- PMID: 20208519
- PMCID: PMC3737736
- DOI: 10.1038/nature08820
Transcription-independent ARF regulation in oncogenic stress-mediated p53 responses
Abstract
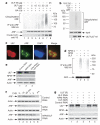
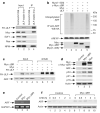
The tumour suppressor ARF is specifically required for p53 activation under oncogenic stress. Recent studies showed that p53 activation mediated by ARF, but not that induced by DNA damage, acts as a major protection against tumorigenesis in vivo under certain biological settings, suggesting that the ARF-p53 axis has more fundamental functions in tumour suppression than originally thought. Because ARF is a very stable protein in most human cell lines, it has been widely assumed that ARF induction is mediated mainly at the transcriptional level and that activation of the ARF-p53 pathway by oncogenes is a much slower and largely irreversible process by comparison with p53 activation after DNA damage. Here we report that ARF is very unstable in normal human cells but that its degradation is inhibited in cancerous cells. Through biochemical purification, we identified a specific ubiquitin ligase for ARF and named it ULF. ULF interacts with ARF both in vitro and in vivo and promotes the lysine-independent ubiquitylation and degradation of ARF. ULF knockdown stabilizes ARF in normal human cells, triggering ARF-dependent, p53-mediated growth arrest. Moreover, nucleophosmin (NPM) and c-Myc, both of which are commonly overexpressed in cancer cells, are capable of abrogating ULF-mediated ARF ubiquitylation through distinct mechanisms, and thereby promote ARF stabilization in cancer cells. These findings reveal the dynamic feature of the ARF-p53 pathway and suggest that transcription-independent mechanisms are critically involved in ARF regulation during responses to oncogenic stress.
Figures


References
-
- Lowe SW, Sherr CJ. Tumor suppression by Ink4a-Arf: progress and puzzles. Curr. Opin. Genet. Dev. 2003;13:77–83. - PubMed
-
- Gil J, Peters G. Regulation of the INK4b-ARF-INK4a tumour suppressor locus: all for one or one for all. Nature Rev. Mol. Cell Biol. 2006;7:667–677. - PubMed
-
- Matheu A, Maraver A, Serrano M. The Arf/p53 pathway in cancer and aging. Cancer Res. 2008;68:6031–6034. - PubMed
-
- Sherr CJ. Divorcing ARF and p53: an unsettled case. Nature Rev. Cancer. 2006;6:663–673. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

