HIV-1 infects multipotent progenitor cells causing cell death and establishing latent cellular reservoirs
- PMID: 20208541
- PMCID: PMC2892382
- DOI: 10.1038/nm.2109
HIV-1 infects multipotent progenitor cells causing cell death and establishing latent cellular reservoirs
Abstract
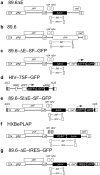
HIV causes a chronic infection characterized by depletion of CD4(+) T lymphocytes and the development of opportunistic infections. Despite drugs that inhibit viral spread, HIV infection has been difficult to cure because of uncharacterized reservoirs of infected cells that are resistant to highly active antiretroviral therapy (HAART) and the immune response. Here we used CD34(+) cells from infected people as well as in vitro studies of wild-type HIV to show infection and killing of CD34(+) multipotent hematopoietic progenitor cells (HPCs). In some HPCs, we detected latent infection that stably persisted in cell culture until viral gene expression was activated by differentiation factors. A unique reporter HIV that directly detects latently infected cells in vitro confirmed the presence of distinct populations of active and latently infected HPCs. These findings have major implications for understanding HIV bone marrow pathology and the mechanisms by which HIV causes persistent infection.
Figures





Comment in
-
Second hideout for HIV-1.Nat Rev Microbiol. 2010 May;8(5):314. doi: 10.1038/nrmicro2359. Nat Rev Microbiol. 2010. PMID: 21080599 No abstract available.
References
-
- Neal TF, et al. CD34+ progenitor cells from asymptomatic patients are not a major reservoir for human immunodeficiency virus-1. Blood. 1995;86:1749–1756. - PubMed
-
- Weichold FF, et al. Neither human immunodeficiency virus-1 (HIV-1) nor HIV-2 infects most-primitive human hematopoietic stem cells as assessed in long-term bone marrow cultures. Blood. 1998;91:907–915. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

