Voltage-dependent gating in a "voltage sensor-less" ion channel
- PMID: 20208975
- PMCID: PMC2826373
- DOI: 10.1371/journal.pbio.1000315
Voltage-dependent gating in a "voltage sensor-less" ion channel
Abstract
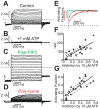
The voltage sensitivity of voltage-gated cation channels is primarily attributed to conformational changes of a four transmembrane segment voltage-sensing domain, conserved across many levels of biological complexity. We have identified a remarkable point mutation that confers significant voltage dependence to Kir6.2, a ligand-gated channel that lacks any canonical voltage-sensing domain. Similar to voltage-dependent Kv channels, the Kir6.2[L157E] mutant exhibits time-dependent activation upon membrane depolarization, resulting in an outwardly rectifying current-voltage relationship. This voltage dependence is convergent with the intrinsic ligand-dependent gating mechanisms of Kir6.2, since increasing the membrane PIP2 content saturates Po and eliminates voltage dependence, whereas voltage activation is more dramatic when channel Po is reduced by application of ATP or poly-lysine. These experiments thus demonstrate an inherent voltage dependence of gating in a "ligand-gated" K+ channel, and thereby provide a new view of voltage-dependent gating mechanisms in ion channels. Most interestingly, the voltage- and ligand-dependent gating of Kir6.2[L157E] is highly sensitive to intracellular [K+], indicating an interaction between ion permeation and gating. While these two key features of channel function are classically dealt with separately, the results provide a framework for understanding their interaction, which is likely to be a general, if latent, feature of the superfamily of cation channels.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






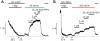
References
-
- Long S. B, Campbell E. B, MacKinnon R. Voltage sensor of Kv1.2: structural basis of electromechanical coupling. Science. 2005;309:903–908. - PubMed
-
- Ahern C. A, Horn R. Focused electric field across the voltage sensor of potassium channels. Neuron. 2005;48:25–29. - PubMed
-
- Bezanilla F. How membrane proteins sense voltage. Nat Rev Mol Cell Biol. 2008;9:323–332. - PubMed
-
- Grabe M, Lai H. C, Jain M, Jan Y. N, Jan L. Y. Structure prediction for the down state of a potassium channel voltage sensor. Nature. 2007;445:550–553. - PubMed
-
- Tombola F, Pathak M. M, Gorostiza P, Isacoff E. Y. The twisted ion-permeation pathway of a resting voltage-sensing domain. Nature. 2007;445:546–549. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

